Articles and Books
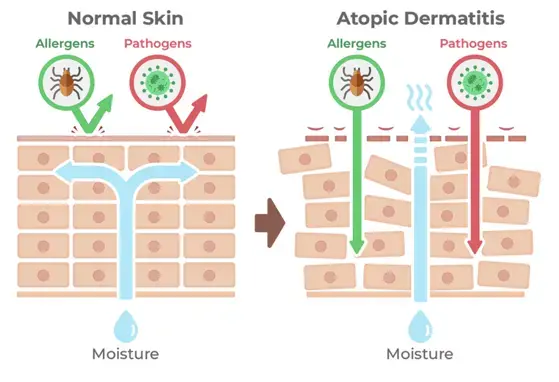
Addressing Atopic Eczema Early in Life to Reduce the Risk of Atopic March
Addressing Atopic Eczema Early in Life to Reduce the Risk of Atopic March
Pivoting the Science of Biotics for Clinical Applications
Pivoting the Science of Biotics for Clinical Applications