Growth, tolerance and safety of an amino acid-based formula supplemented with two human milk oligosaccharides in infants with moderate-to-severe cow’s milk protein allergy
This is the first study to evaluate infants with moderate to severe Cow’s Milk protein Allergy (CMPA) fed an Amino Acid based formula (AAF) supplemented with two HMO. The infants achieved normal growth, with some catch-up growth. Apart from a small number of non-serious gastrointestinal adverse events, the formula was tolerated well and had an excellent safety profile.
Background
We aimed to assess if an amino acid-based formula (AAF) supplemented with two human milk oligosaccharides (HMO) supports normal growth and is well tolerated in infants with moderate-to-severe cow’s milk protein allergy (CMPA).
Method
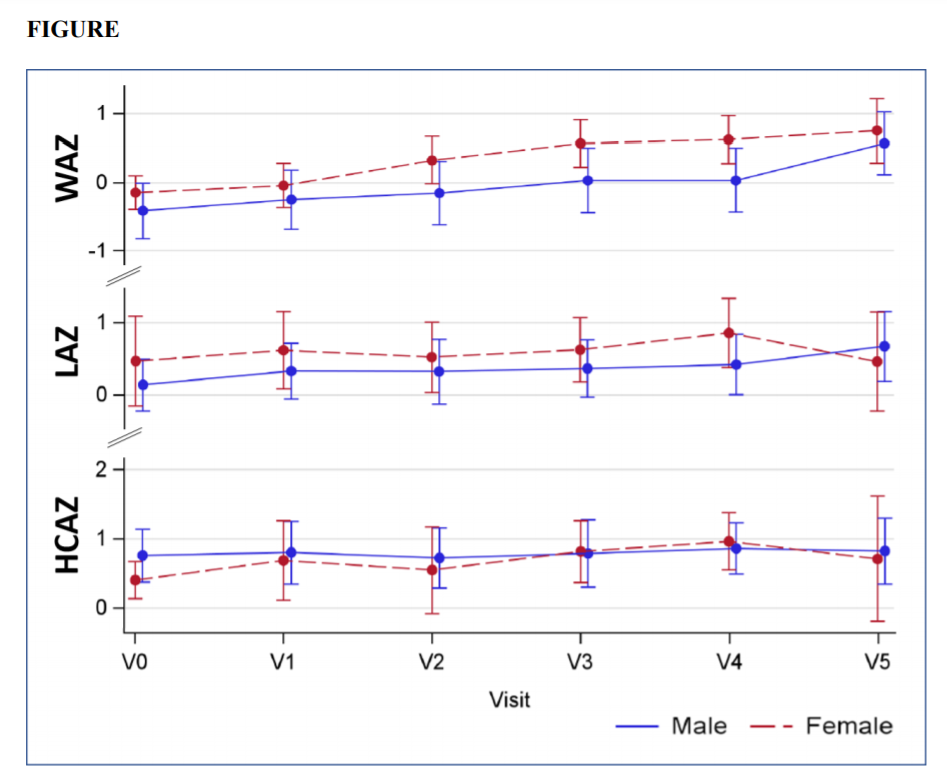
This open-label, non-randomized, single-intervention, multi-centre trial was conducted at 6 paediatric sites in Australia. Full-term infants aged 1–8 months with moderate-to-severe CMPA who had failed a trial of extensively hydrolysed formula (EHF) or rice-based formula (RBF), and those who required AAF as first-line treatment, were enrolled. Study formula was an AAF supplemented with two HMO, 2’fucosyl-lactose (2’FL) and lacto-N-neotetraose (LNnT). Infants were given the formula for 4 months (principal study period) and continued up to 12 months of age, as required. Infants received a cow’s milk protein-free, complementary diet from 4–6 months. Weight, length and head circumference were measured monthly from enrolment (V0) to the 4-month follow-up (V4), as well as at 12 months of age (V5). Weight-for-age, length-for-age and head circumference-for age Z-scores (WAZ, HAZ, HCAZ) were calculated based on the WHO growth reference, and summary statistics provided for each timepoint. Tolerance and safety were assessed based on adverse events reporting throughout the trial.
Results
Of 34 infants screened, 32 were enrolled and 29 completed the trial to V4. Mean age at V0 was 18.6 ± 8.0 weeks (range 4–37); 20 [62.5%] male). At the time of enrolment, 16 (50%) and 3 (9.4%) had failed a trial of EHF or RBF, respectively; 13 (40.6%) infants were transitioned from another AAF. During the principal study period (V0 to V4), the mean WAZ increased from -0.31 to +0.28 (delta WAZ +0.59). Similarly, the mean LAZ increased from +0.26 to +0.60 (delta LAZ +0.34) and the mean HCAZ from +0.63 to +0.91 (delta HCAZ +0.28); Figure. Overall, there were 232 adverse events (AE) of which 192 (82.8%) were classified as mild, 37 (15.9%) as moderate and 3 (1.3%) as severe. All moderate or severe AE were deemed ‘unrelated’ or ’unlikely related’ to the study formula. The formula was discontinued in 2 (6.3%) infants due to mild, non-serious AE (gastro-oesophageal reflux [n=1]; loose stools, flatulence and decreased feeding [n=1]).
Conclusion
Infants with moderate-to-severe CMPA fed the study formula with two HMO achieved normal growth, with some catch-up growth. Apart from a small number of non-serious gastrointestinal AE, the formula was tolerated well and had an excellent safety profile.
If you liked this post you may also like