Low-Protein Formula Slows Weight Gain in Infants of Overweight Mothers
Low-Protein Formula Slows Weight Gain in Infants of Overweight
Mothers
Jaime Inostroza, Ferdinand Haschke, Philippe Steenhout, Dominik Grathwohl, Steven E. Nelson, and Ekhard E. Ziegler
Key Words: anthropometry, biomarkers, body composition, growth, low- protein formula, overweight mothers
ABSTRACT
Objectives: Infant formulas provide more protein than breast milk. High protein intakes, as well as maternal obesity, are risk factors for later obesity. The present study tested whether a formula with lower protein content slows weight gain of infants of overweight mothers (body mass index [BMI] > 25 kg/m2).
Methods: In a randomized double-blind study infants of overweight mothers received from 3 months an experimental (EXPL) formula with 1.65 g of protein/100 kcal (62.8 kcal/100 mL) and containing probiotics, or a control (CTRL) formula with 2.7 g of protein/100 kcal (65.6 kcal/100 mL). Breast-fed infants were studied concurrently. Primary assessment was between 3 and 6 months, although formulas were fed until 12 months. Biomarkers of protein metabolism (blood urea nitrogen, insulin growth factor-1, insulinogenic amino acids) were measured.
Results: Infants fed the low-protein EXPL formula gained less weight between 3 and 6 months (1.77 g/day, P ¼ 0.024) than infants fed the CTRL formula. In the subgroup of infants of mothers with BMI > 30 kg/m the difference was 4.21 g/day (P ¼ 0.017). Weight (P ¼ 0.011) and BMI (P ¼ 0.027) of EXPL infants remained lower than that of CTRL infants until 2 years but were similar to that of breast-fed infants. Blood urea nitrogen, insulin growth factor-1, and insulinogenic amino acids at 6 months were significantly lower in EXPL compared with CTRL.
Conclusions: A low-protein formula with probiotics slowed weight gain between 3 and 6 months in infants of overweight mothers. Weight gain and biomarkers were more like those of breast-fed infants.
Protein needs of infants decrease appreciably during the first year of life (10). During the first few months, breast milk alone and subsequently breast milk with complementary foods are presumed to meet the protein needs of infants. The protein content of human milk, which may be as high as 2.09 g/100 kcal in the first month after birth, is 1.28 g/100 kcal at 3 to 4 months (11) and approximately 1.24 g/100 kcal by 9 to 12 months. This suggests that formulas fed after 3 months should contain no less than 1.30 g/100 kcal of a high-quality protein. The lower regulatory limit for protein content of formulas (0– 12 months) in the European Union (EU) and the United States is 1.8 g/100 kcal. In actuality, the protein content of formulas typically exceeds these levels, especially in the case of follow-on formulas. In the European Obesity Project (12), the protein concentration of the lower-protein formula (2.20 g/100 kcal) fed from 5 months on exceeded the lower limit in EU countries. Protein intakes in the later parts of infancy have been found in several localities to be high and to exceed required intakes (13–16). Epidemiologic evidence links high protein intakes in infancy to obesity in childhood (16– 19). Also, high protein intakes have been shown in prospective studies to lead to increased weight gain and higher adiposity in infancy (12) and childhood (20). Lower protein intake from breast milk than from formula may be among the reasons why breast-fed (BF) infants are at lower risk for obesity later in life, as the preponderance of the evidence indicates (7,21–23). For all of these reasons, reducing the protein intake during infancy may reduce the risk of later obesity.
The present study tested a bovine whey-based formula with a protein content of 1.65 g/100 kcal, which is below the regulatory lower limit in Europe and the United States. Because protein levels <1.80 g/100 kcal have not been studied before, we reduced the protein level by only 9% below the regulatory level. The formula was fed after 3 months of age. The offspring of overweight and obese women are at increased risk for overweight later in life (7,24 – 31) and show accelerated growth already during infancy (32). Therefore, any measure that could reduce the risk of later obesity would be of particular importance for infants born to overweight mothers.
METHODS
Study Design
The study was designed to test the hypothesis that a formula with a protein content of 1.65 g/100 kcal and a caloric density of 62.8 kcal/100 mL that contains added probiotics (Lactobacillus PR and Bifidobacterium lactis) (EXPL [experimental] formula) leads to slower growth between 3 and 6 months of age than a formula with a protein content of 2.70 g/100 kcal and standard caloric density of 65.6 kcal/100 mL without probiotics (CTRL [control] formula). Furthermore, it was hypothesized that the lower-protein formula would lead to biomarkers of protein metabolism that were closer to those of BF infants. A secondary objective of the study was to establish that the formula with protein content 1.65 g/100 kcal supports normal growth. The hypothesis was tested in a double-blind trial in which formula- fed infants were at 3 months randomly assigned to 1 of the study formulas, which was to be fed exclusively until 6 months and with complementary foods until 12 months. A reference group of BF infants was studied in identical fashion. Growth was monitored until 24 months and formula consumption was determined periodically until 9 months. The study protocol was reviewed and approved by the ethical committee of Regional Health Service, IX Region de la Araucan´ıa, Chile.
The age period from 3 to 6 months was treated as the primary study period, with growth during that period designated as the primary outcome because during that period the study formulas were the near-exclusive source of nutrients. Absence of complementary foods was considered important for an evaluation of the adequacy of the formulas.
Sample Size Calculation
Sample size was estimated assuming that a difference in weight gain of 2 g/day is clinically relevant. According to the World Health Organization (WHO) Child Growth Standards (33), weight gain between 3 and 6 months averages 17 g/day, with a standard deviation of approximately 4 g/day (boys and girls). To detect a difference of 2 g/day with a type I error (a) of 5% (2-sided test) and power of 80%, 64 infants were needed in each arm of the formula trial. With an expected dropout rate of approximately 30%, a total of 182 formula-fed infants needed to be enrolled. For the BF reference group similar considerations indicated that 91 infants needed to be enrolled. As the study progressed, it became clear that the actual dropout rate was <30% and enrollment was stopped when it could be expected that at least 64 infants per group would reach 6 months.
Subjects
Pregnant women attending the maternity facilities associated with the Universidad de La Frontera were, under the supervision of the principal investigator (J.I.), screened. If their (self-reported and/or medical records) prepregnancy body mass index (BMI) was >25 kg/m2, they were informed about the study. Medical and other information about the mothers was obtained from hospital records or from the mothers directly. At birth the infant was assessed and was offered study participation if he or she met the study inclusion criteria (birth weight >2500 g and <4800 g, gestational age 37 weeks and <42 weeks) and did not meet the exclusion criteria (weight <5th percentile for gestational age, maternal diabetes, including gestational-onset diabetes, >5 cigarettes per day during pregnancy, use of illicit drugs, or presence of chronic inflammatory condition). Infants with congenital illnesses or malformations that may affect growth were excluded, as were infants who required hospitalization for >2 days.
Of 330 infants screened, 305 were enrolled at birth (N 302) or between 5 and 31 days of age (N 3). Mothers provided written informed consent at the time of enrollment. Most infants were BF when they left the hospital. All of the mothers received breast- feeding consultation during their visits in the study center. At 1.5 months of age, approximately 80% of infants were exclusively or partially BF. Whenever mothers chose to discontinue breast- feeding, a whey-based infant formula (NAN 1; protein 1.8 g/100 kcal, 67 kcal/100 mL [Nestle´ Ltd, Vevey, Switzerland]) was provided. At 3 months of age, infants who were predominantly BF (no more than 1 formula feeding per day) were assigned to the BF reference group (n 76). Predominantly formula-fed infants were randomly assigned to 1 of the study formulas, that is, to EXPL formula (n 86) or CTRL formula (n 86).
The flow of subjects in the intention-to-treat (ITT) population through 12 months is shown in Figure 1. Formula-fed infants were withdrawn from the study because mothers objected to blood draws (n 2), infants did not accept study formulas (n 2), and mothers wished to continue mixed feeding (formula breast- feeding) (n 14). An additional 12 formula-fed infants left the study for reasons unrelated to the study. Although somewhat more EXPL infants (n 20) left the study before 6 months than CTRL infants (n 10), there was not a single cause that explained the difference. Four BF infants were withdrawn because mothers wished to continue mixed feeding. A total of 170 infants (56 BF, 50 EXPL, and 64 CTRL) in the ITT sample were studied up to 24 months. In the per-protocol (PP) sample there were at 6 months 55 infants in EXPL and 68 in CTRL; at 12 months there were 47 in EXPL and 60 in CTRL.
Study Feedings
Composition of the study formulas is indicated in supplementary Table S1 (http://links.lww.com/MPG/A304). Both formulas were produced especially for the present trial. The formulas differed in protein content, which was 1.65 g/100 kcal in the EXPL formula and 2.70 g/100 kcal in the CTRL formula. Protein was provided by intact bovine milk proteins with a whey- to-casein ratio of 60:40. Details of the protein composition in the EXPL formula are presented in Table S1. Because of reported beneficial effects that probiotics confer on infants (34 – 36), the EXPL formula contained 2 10 cfu/g formula powder of each of the probiotic strains Lactobacillus PR and B lactis (Bb12); the CTRL formula did not contain probiotics. Levels of minerals, vitamins, and trace elements in the study formulas corresponded to the recommendations of the Codex Alimentarius (37) for infant and follow-up formulas. The formulas were provided in powder form. Parents were requested not to feed any other formula and not to start complementary foods until 6 months. The study formulas were provided free of charge until 12 months. The tins were labeled with color codes (2 colors for each formula); the identity of the formulas was unknown to the parents, study staff, and investigators, and was known only to the manufacturer. BF infants could at study entry (3 months) receive up to 1 formula feeding per day. Parents were requested to withhold complementary feedings until 6 months. After 6 months, whenever the mother chose to discontinue breast-feeding, a commercially available follow-up formula (NAN 2) was provided until 12 months of age that had a protein content of 2.4 g/100 kcal and a caloric density of 67 kcal/100 mL.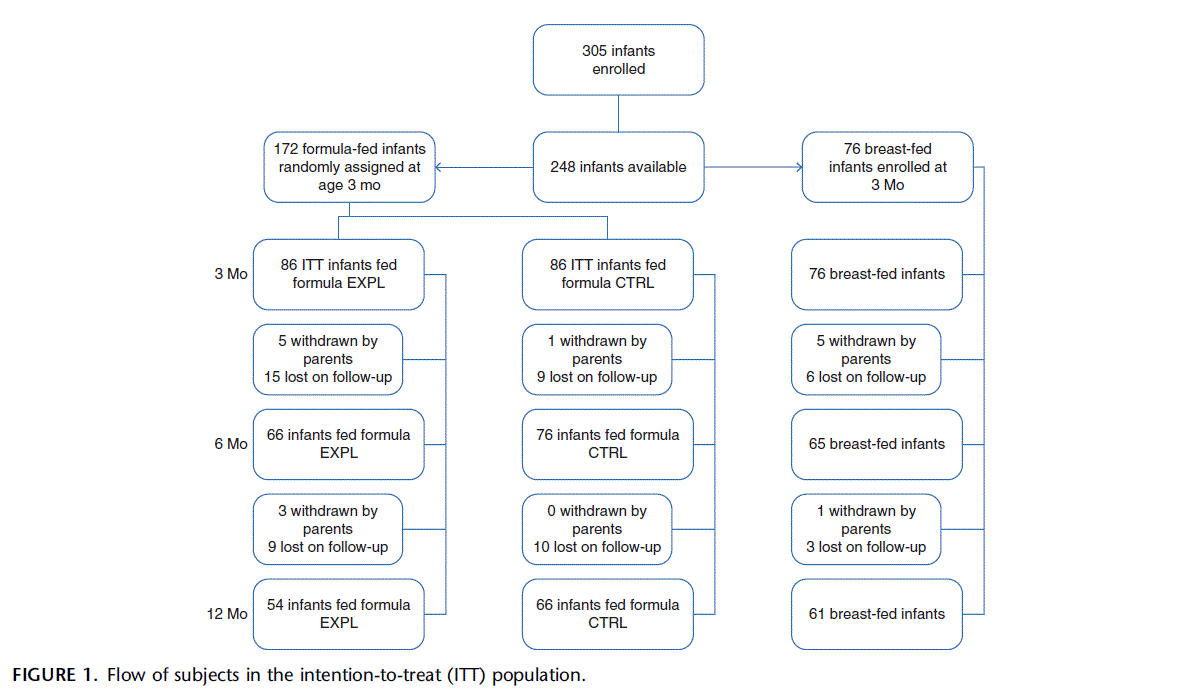
Study Procedures
After enrollment infants were seen within 5 days of age 1.5 months and subsequent study visits took place within 7 days of ages 3, 4, 6, 9, and 12 months and within 14 days of age 24 months. At birth, anthropometric data were obtained from hospital records. At study visits, anthropometry was performed and an interval medical history was obtained.
At the 3-month visit, formula-fed infants were randomly assigned to EXPL formula or CTRL formula using the Internet- based randomization system, TrialSys. Stratification factors were sex, ethnicity (white/other), prepregnancy BMI of the mother (25– 30, >30 kg/m2), and type of feeding between 1.5 and 3 months (formula exclusively or formula and breast). At study visits, a supply of study formula was dispensed that was expected to last until the next study visit.
Anthropometry: During study visits, weight without clothes was determined to the nearest 10 g using calibrated electronic scales (Sartorius, Gottingen, Germany). Recumbent length was measured by 2 measurers using a measuring board with fixed head board and movable foot board (infant stadiometer) and was recorded to the nearest 1 mm. Head circumference was measured using a non- stretchable measuring tape to the nearest 1 mm. All measurements were obtained in duplicate and the average was used. Body com- position measurements at 12 to 13 months were performed using a DXA Lunar Prodigy Advance with software EnCore version 13.6 (both GE Healthcare, Madison, WI).
Dietary intake: Intake of formula (quantitative) and consumption of complementary foods (semiquantitatively) were recorded by the parents on hand-held diaries for 3 days before the visits at 3, 4, 6, and 9 months of age. At study visits, records were checked for completeness and ambiguities were resolved.
Serum (plasma) biomarkers: Venous blood was obtained during visits at 3, 6, and 12 months. Blood was drawn >1.5 hours after the previous meal. Blood was drawn into heparinized (for amino acid determinations) and EDTA-containing (for ghrelin determinations) tubes or plain tubes (for all other determinations). Determinations were performed by the Central Laboratory in Temuco unless noted otherwise. Determinations of insulin growth factor-1, C-peptide, and leptin were performed by ELISA using kits from ALPCO (ALPCO Diagnostics, Salem, NH). Determination of insulin was performed by Microplate Enzymatic Immuno-Assay (Insulin Assay; Axsym, Abbott, Abbott Park, IL). Ghrelin was determined by enzyme immunometric assay (ALPCO) at the Nestle´ Research Center, Lausanne, Switzerland. Amino acids were deter- mined using the EZ:Faast-Free Amino Acid kit (Phenomenex, Torrance, CA) and gas chromatography at Fleury Laboratory, Sa˜o Paulo, Brazil.
Data Processing and Statistical Analysis
Data were analyzed on an ITT basis and also on a PP basis. Results based on ITT analysis are presented unless otherwise specified. Inclusion in the PP sample required that infants consumed study formula from 3 to 6 months exclusively, which was defined as no breast-feeding indicated in the diaries at 4 and 6 months, nonstudy formula <1 bottle/week or fewer than 3 full consecutive days, and consumption of solid foods <4 teaspoons/day until 4 months and <1 serving/day thereafter. Subjects were also excluded from PP evaluation if they were hospitalized for more than 3 days or received treatment with oral corticosteroids for >15 days.
Weight gain (g/day) between 3 and 6 months (primary out- come variable), 6 and 12 months, 12 and 24 months, 3 and 12 months, and 3 and 24 months was calculated as the difference in weight divided by the exact number of days between measurements. Weight gain between 3 and 6 months of formula-fed infants was also calculated separately for infants with weight >75th percentile at 3 months, for infants whose mother had a BMI > 30 kg/ m2, and for infants with both of these characteristics. Differences between groups were compared by t test (primary outcome only) and also by ANCOVA correcting for infant weight at 3 months, maternal BMI (kg/m2), sex (male/female), ethnicity (white/other), antibiotic use, and complementary food before 6 months. Decisions on covariates were made before the code was broken.
z scores for weight, length, BMI, and head circumference at 3 to 24 months were derived based on the WHO 2006 Child Growth Standards (38). Baseline values (3 months) of z scores were compared using ANCOVA correcting for maternal BMI (kg/m2), sex (male/female), and ethnicity. Longitudinal comparison of weight, length, BMI, and head circumference and of the corresponding z scores (4–24 months) were performed by a mixed model to test for different time trends (39). Fixed effects were the correction factors used in the ANCOVA along with visits, treatment, and visit treatment. Random effect was subject. The percentage of infants in EXPL and CTRL with weight >90th percentile of the WHO Standards at 3, 6, and 12 months was compared by calculating odds ratios (ORs) and 95% confidence intervals (CIs) by logistic regression.
Serum biomarkers that showed lognormal distribution were analyzed after logarithmic transformation, but data are presented as arithmetic means and standard deviations. Only PP data are presented because biochemical parameters are strongly influenced by nutritional intake. Plasma amino acid concentrations were compared by ANCOVA correcting for maternal BMI. Comparisons of biomarkers were performed by ANCOVA correcting for values at 3 months. Body composition data were compared by the 2-sample t test. Intakes (volume, energy) were compared by ANCOVA correcting for intakes at 3 months.
RESULTS
Characteristics of mothers and infants are presented in supplementary Table S2 (http://links.lww.com/MPG/A305). Study infants were born between October 2007 and September 2009. Differences between groups were small and not statistically significant, except that mothers of BF infants were less likely to smoke during pregnancy but were more likely to consume alcohol than mothers of formula-fed infants. Infant anthropometric data at birth were similar for all 3 groups.
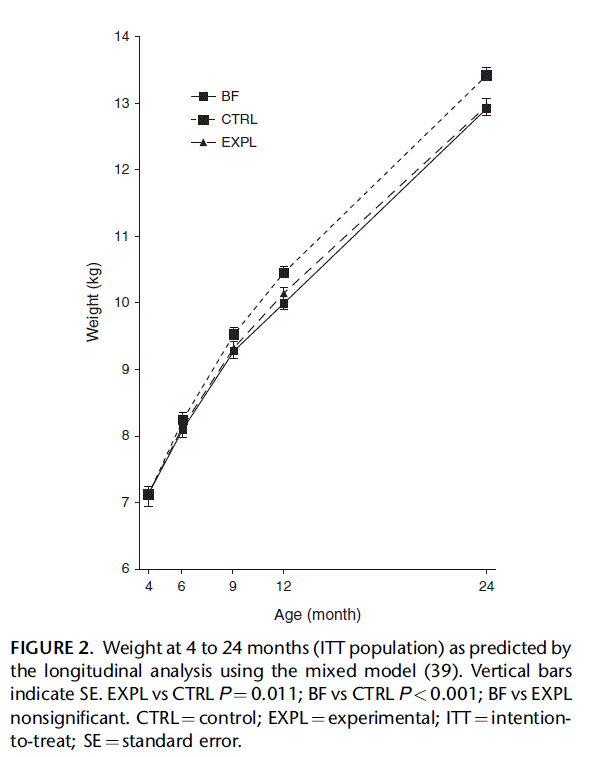
Weight gain between 3 and 6 months, the primary study outcome, was significantly lower in EXPL than in CTRL. The difference was 1.77 g/day (95% CI 3.29 to 0.24, P 0.024, t test) in the ITT sample (Table 1, top panel). In the PP sample (data not shown) the difference was 1.85 g/day (95% CI 3.46 to 0.24, P 0.025). After correcting for covariates by ANCOVA, the difference remained significant for the period 3 to 6 months (lower panel of Table 1). The difference in weight gain between 3 and 6 months was almost entirely explained by the strong effect in infants whose weight was >75th percentile at 3 months. In that subgroup (EXPL N 22, CTRL N 28), the difference was 3.70 g/day (95% CI 6.69 to 0.70, P 0.016). In the subgroup of infants whose mothers had a BMI > 30 kg/m2 (EXPL N 21, CTRL N 21), the difference was 4.21 g/day (95% CI 7.75 to 0.81, P 0.017). In infants with both characteristics (EXPL N 6; CTRL N 7), the difference was as large as 8.34 g/day (CI 16.05 to 0.63, P 0.035). Weight gain of EXPL was not significantly different from that of BF throughout the study, but weight gain of CTRL was higher than that of BF for most time intervals (Table 1). Weight predicted by the longitudinal analysis is shown in Figure 2. Overall, in the longitudinal analysis the difference in weight between EXPL and CTRL was statistically significant in both the ITT and the PP samples (P 0.022). Weight-for-age z scores (Table 2) of EXPL and CTRL were not significantly different at 3 months, but z scores of BF were significantly higher. After 3 months weight-for-age z scores began to differ between EXPL and CTRL; the difference was statistically significant at 9 months and continued to increase until 24 months. The differences in z scores at 12 and 24 months corresponded to weight differences of 316 and 446 g, respectively. Differences between EXPL and BF between 6 and 24 months were not significant. On the contrary, differences between CTRL and BF were statistically significant between 9 and 24 months of age. The percentage of infants whose weight was >90th percentile of the WHO standards was similar in EXPL and CTRL at 3 months (OR 1.0, 95% CI 0.4– 2.5) but at 6 and 9 months it was significantly lower in EXPL than in CTRL. The percentages at 6 months were 10.6% in EXPL and 22.4% in CTRL FIGURE 2. Weight at 4 to 24 months (ITT population) as predicted by the longitudinal analysis using the mixed model (39). Vertical bars indicate SE. EXPL vs CTRL P ¼ 0.011; BF vs CTRL P < 0.001; BF vs EXPL nonsignificant. CTRL ¼ control; EXPL ¼ experimental; ITT ¼ intention- to-treat; SE ¼ standard error. (OR 5.3, 95% CI 1.2– 23.5) and at 12 months were 18.5% and 31.8% (OR 3.6, 95% CI 1.1–11.2). The number needed to treat was 8, meaning that 8 infants would need to be fed the EXPL formula to prevent 1 formula-fed infant from exceeding the 90th percentile at 6 or 12 months.
Length-for-age z scores (supplementary Table S3, http:// links.lww.com/MPG/A306) were at 3 months significantly lower in the 2 formula groups compared with the BF group. Subsequently, with correction for length at 3 months, differences were not significant between 6 and 24 months. Mean head circumference z scores (data not shown) were above the corresponding WHO standards at all ages for all groups and standard deviations were <1. Differences in head circumference z scores between EXPL and BF were not significant. Length and head circumference, as predicted by the longitudinal analysis, showed no differences between the 3 groups (data not shown).
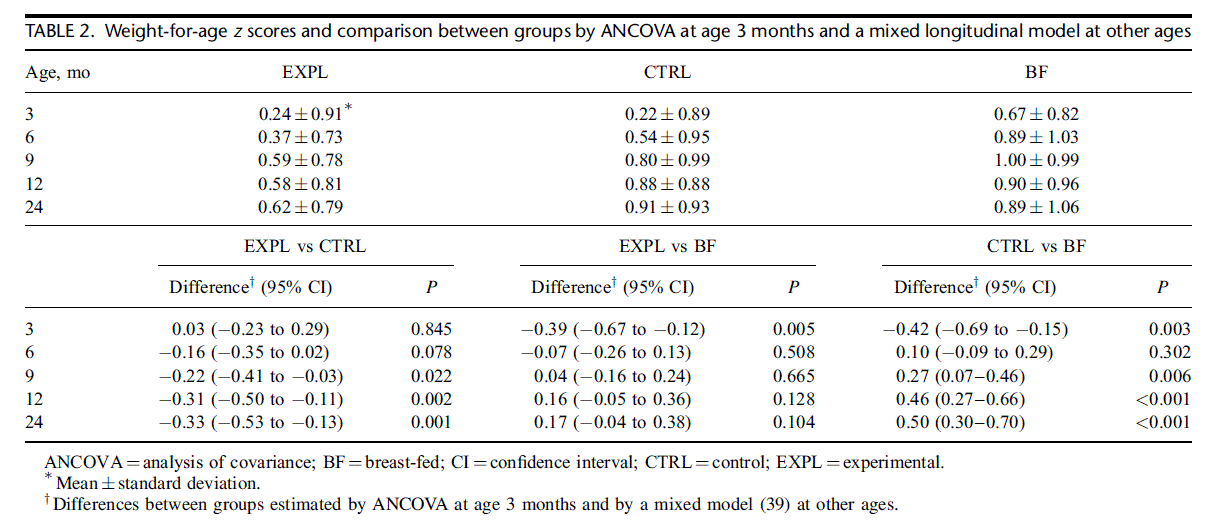
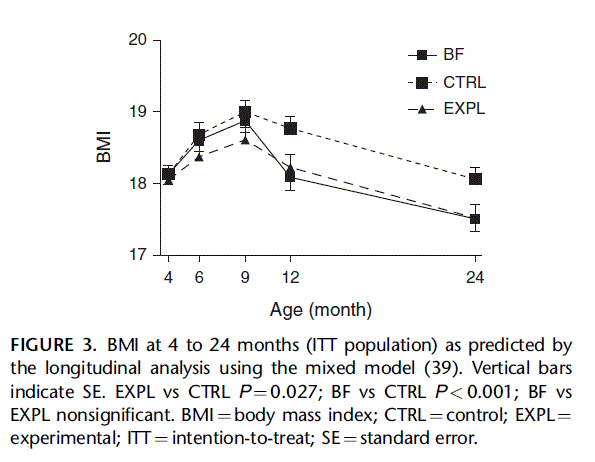
BMI predicted by the longitudinal analysis (Fig. 3) was significantly lower in EXPL and BF than in CTRL (EXPL vs CTRL P 0.027, PP). BMI of EXPL and BF did not differ significantly and, at 24 months, were almost identical. BMI-for- age z scores at 3 months (supplementary Table S4, http://links.lww. com/MPG/A307) were not significantly different in EXPL com- pared with CTRL; however, at subsequent ages, BMI z scores were lower in EXPL than in CTRL; and at 12 and 24 months, the difference reached statistical significance. Also, at 12 and 24 months, BMI z scores of BF were significantly lower than those of CTRL. Notably, mean BMI z scores of all 3 groups were greater than zero and increased between 3 and 24 months, indicating progressively greater BMI compared with the WHO standards.
Results for serum biomarkers are summarized in Table 3. Blood urea nitrogen showed significant differences only at 6 months when concentration was lowest in BF and highest in CTRL, with EXPL showing intermediate levels. At 12 months, blood urea nitrogen concentrations were no longer significantly different. Insulin growth factor-1 concentration at 6 months was significantly lower in EXPL and BF compared with CTRL. Plasma concentrations of essential amino acids and tyrosine at 6 months of age are presented in supplementary Table S5 (http://links.lww.com/MPG/ A308). Concentrations of the insulinogenic amino acids leucine, isoleucine, and valine were significantly lower in EXPL than in CTRL, but none of the other amino acids showed significant differences between EXPL and CTRL. In BF infants concentrations of most amino acids were appreciably lower than in formula-fed infants. The exceptions were histidine and threonine, where concentrations were similar, and glutamine and serine, where concentrations were higher in BF than in the formula groups.
Body composition was determined in a subset of subjects at 12 months of age. Data are summarized in supplementary Table S6 (http://links.lww.com/MPG/A309). Differences in fat mass and in lean mass were not statistically significant. Bone mineral content was lower in EXPL than in CTRL, but when expressed per unit of lean body mass, the difference was not statistically significant. FIGURE 3. BMI at 4 to 24 months (ITT population) as predicted by the longitudinal analysis using the mixed model (39). Vertical bars indicate SE. EXPL vs CTRL P ¼ 0.027; BF vs CTRL P < 0.001; BF vs EXPL nonsignificant. BMI ¼ body mass index; CTRL ¼ control; EXPL ¼ experimental; ITT ¼ intention-to-treat; SE ¼ standard error. Formula intake (milliliters per day) (supplementary Table S7, http://links.lww.com/MPG/A310) as recorded by parents was not significantly different in EXPL compared with CTRL between 4 and 9 months; however, calculated energy intakes were almost identical at 4 and 6 months. Protein intakes showed large and highly significant differences between EXPL and CTRL.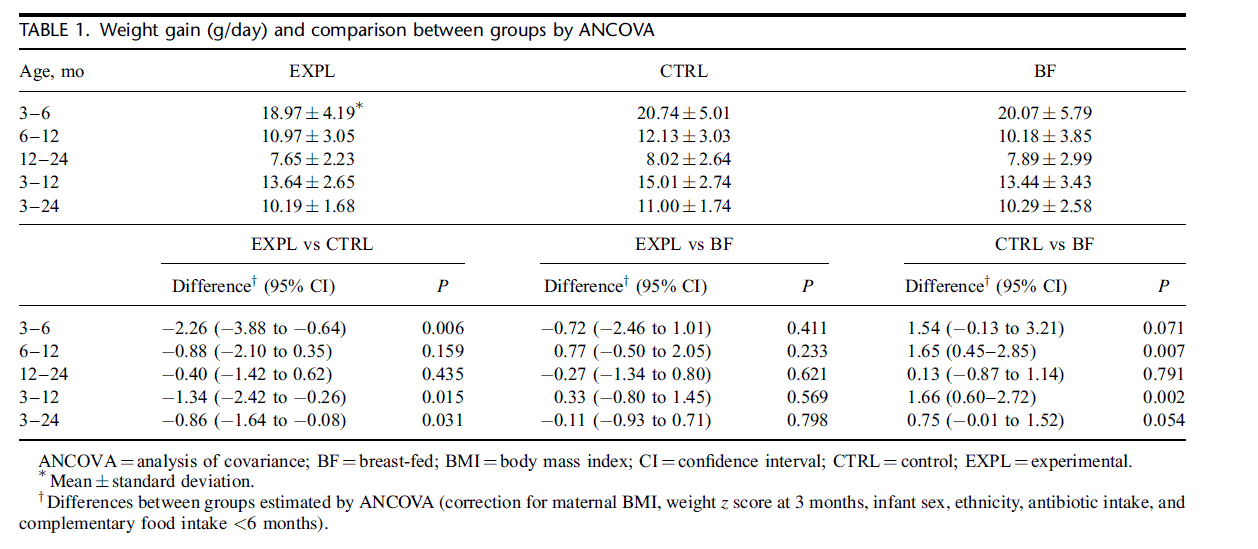
DISCUSSION
The dual purpose of the present study was to determine whether in infants of overweight mothers a whey-based formula with lower protein and energy content decreases weight gain, and whether the lower protein intake still meets the needs of infants. The study was conducted in infants of overweight mothers because these infants are at increased risk for overweight later in life and may particularly benefit from slowed growth.
The experimental formula used in this randomized double-blind trial had a protein content of 1.65 g/100 kcal, which was lower than that of currently available formulas and lower than the regulatory limits in the EU and the United States. The protein content was still well above 1.30 g/100 kcal, which is the protein content considered adequate for infants beyond 3 months of age (10,11) and the amino acid profile of the formula corresponded to that of human milk. Formulas with protein content <1.8 g/100 kcal have not been evaluated in infants of this age.
The main finding of the present study was that feeding the EXPL formula had the effect of slowing down rapid weight gain between 3 and 6 months compared with the CTRL formula. The finding could also be interpreted as showing that the higher protein intake provided by the CTRL formula was accelerating weight gain. Either way, the EXPL formula prevented accelerated weight gain. It is likely that the growth-slowing effect of the EXPL formula was because of its lower protein content. The presence of the 2 probiotic strains (B lactis and Lactobacillus PR) was highly unlikely to have had an effect on weight gain. Other studies and committee statements have clearly shown that the 2 selected probiotic strains did not affect growth (40–42). Probiotics were added to the EXPL formula because of the reported beneficial effects they confer on infants (34–36). Similarly, the small difference in energy density of 2.8 kcal/100 mL was highly unlikely to have affected growth as recorded energy intakes were nearly identical.
It is of note that the preventive effect of the EXPL formula occurred mainly in infants of obese women and in infants with weight >75th percentile. These are the groups of infants presumed to be at particularly high risk of later obesity. Infants born to overweight mothers are prone to show accelerated weight gain during infancy (32). Growth of infants fed EXPL was similar to that of BF infants. Biomarkers of protein metabolism indicated that the amount of protein provided by the CTRL formula exceeded the needs of the infants by a larger margin than the EXPL formula. In this regard our results are consistent with the findings of the European Obesity Project (43).
The other main finding of the present study was that the EXPL formula with a protein concentration 9% below the regulatory limits supported normal growth of infants from 3 months on. Given that the protein content of the formula was higher than that of breast milk, the finding was not surprising. Nevertheless, it is an important finding because it establishes that a formula with this concentration of a high-quality protein provides an intake that appears to be adequate in every respect. The judgment of adequacy rests on the observed growth performance, which showed that weight-for-age z scores of all 3 groups were at all ages significantly greater than zero, meaning that the average weight of study infants was greater than the average weight of the WHO standards. EXPL infants were never at risk for having weight-for-age z scores < 2.0, which is the WHO definition of malnutrition. Negative values for mean z scores for length indicated that average length of EXPL and CTRL was lower than the WHO mean length, whereas the length of BF infants tended to be greater than the WHO mean. The length of infants in EXPL tracked the respective WHO z score channel (38) until 24 months. Standard deviations of length z scores in EXPL infants at 6, 9, and 12 months were <1. The study therefore showed that EXPL infants were not at risk for inadequate length gain.
The judgment of adequacy also rests on metabolic parameters, which were close to those of the BF group and were consistent with the notion that protein intakes were slightly above those of BF infants. In addition, body composition measurements at 12 months of age indicated similar fat mass and fat-free mass in infants fed the EXPL formula and BF infants.
High intakes of protein that are commonly observed among older infants (13– 16) have been linked to increased risk of obesity in childhood in epidemiologic studies (16–19). Direct evidence for the growth-stimulating effect of high-protein feedings has been provided by the prospective, randomized trial conducted in the European Obesity Project (12). The feeding of a formula with high protein content (4.60 g/100 kcal) led to increased weight with evidence of increased adiposity. The protein content of the high- protein formula used in that trial (12) was representative of the traditional high protein content of formulas for older infants (follow-on formulas). Protein concentrations of formulas need to be somewhat higher than the protein concentration of human milk because the quality of formula protein may be somewhat less than that of human milk protein. The concentration of 1.65 g/100 kcal was still well above the required level of 1.30 g/100 kcal and could thus be expected to support normal growth. The protein content of infant formulas has successfully been lowered to 1.80 to 1.90 g/100 kcal; this has been shown to support normal growth in the first 4 months of life (44–46).
The present study was conducted among infants at increased risk of obesity later in life because of having an overweight or obese mother (7,24–32). It is therefore necessary to exercise caution before generalizing the present findings to all infants regardless of the weight status of their mothers; however, the fact that our findings agree closely with those of Koletzko et al (12) suggests that the present findings may be generalizable. Nevertheless, to establish safety and efficacy of the low-protein formula for the general infant population, studies are needed of infants of nonobese mothers conducted in countries other than Chile.
The study had some limitations. It would have been desirable for both formulas to have the exact same caloric density and for each formula to contain probiotics. As mentioned above, however, calorie intakes were nearly identical, and the presence of the 2 probiotic strains was highly unlikely to have affected growth, the primary outcome.
There clearly is a need for effective interventions capable of decreasing obesity in childhood (47). Educational interventions have so far been only moderately successful (48). The promotion of breast-feeding undoubtedly is an important weapon in efforts to curtail obesity. The use of low-protein feedings after 3 months, like the one used in the present study, expands the scope of possibilities for intervention. Whether the slowing of growth in the short term translates to a reduction of obesity risk in the long term remains to be determined.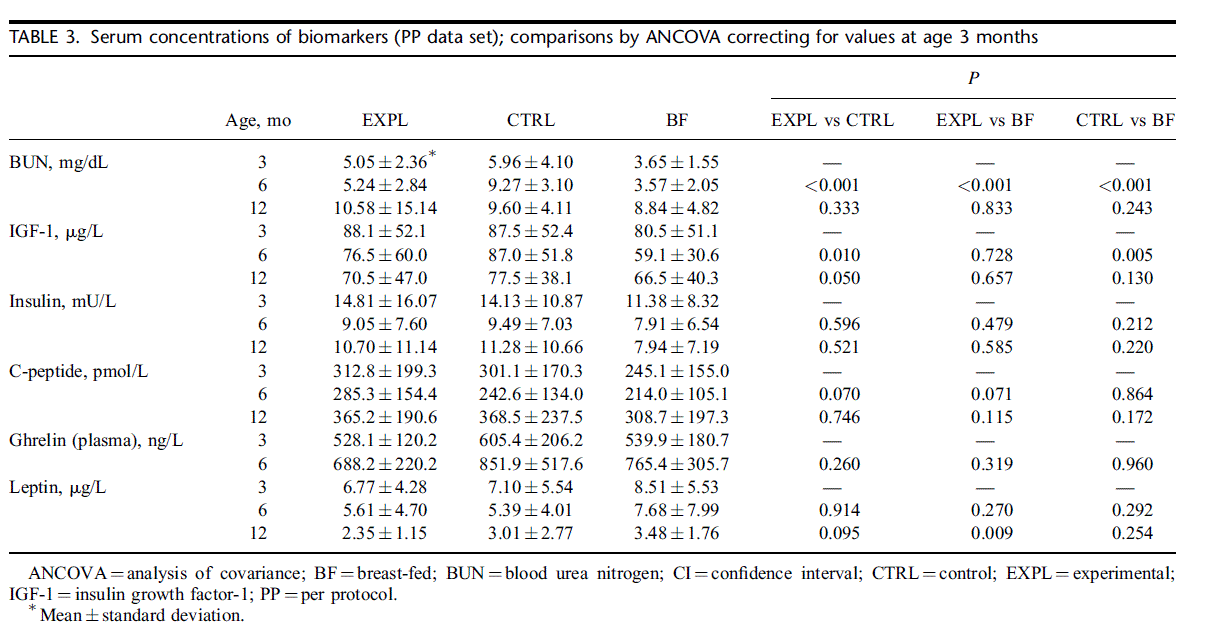
Acknowledgments: The authors acknowledge the substantial contributions that were made to the conduct of the study by Jorge Sapunar, MD; Ruth Prieto, MW; Ana M. Vinet, MD; Jacqueline Fierro, MD; Andres Roman, MD; and Eduardo Hebel, MD. The authors also thank Franc¸oise Chauffard and Sara Colombo-Mottaz for managing the study and Emilie Ba for data management.
REFERENCES
1. Eid EE. Follow-up study of physical growth of children who had excessive weight gain in first six months of life. Br Med J 1970; 2:74–6.
2. Stettler N, Zemel BS, Kumanyika S, et al. Infant weight gain and childhood overweight status in a multicenter cohort study. Pediatrics 2002;109:194–9.
3. Stettler N, Kumanyika S, Katz SH, et al. Rapid weight gain during infancy and obesity in young adulthood in a cohort of African Amer- icans. Am J Clin Nutr 2003;77:1374–8.
4. Baird J, Fisher D, Lucas P, et al. Being big or growing fast: systematic review of size and growth in infancy and later obesity. BMJ 2005; 331:929–35.
5. Demerath EW, Reed D, Choh AC, et al. Rapid postnatal weight gain and visceral adiposity in adulthood: the Fels longitudinal study. Obesity 2009;17:2060–6.
6. Leunissen RWJ, Kerkhof GF, Tijnen T, et al. Timing and tempo of first- year rapid growth in relation to cardiovascular and metabolic risk profile in early adulthood. JAMA 2009;301:2234–42.
7. Weng SF, Redsell SA, Swift JA, et al. Systematic review and meta- analyses of risk factors for childhood overweight identifiable during infancy. Arch Dis Child 2012;97:1019–26.
8. Adair LS, Fall CHD, Osmond C, et al. Associations of linear growth and relative weight gain during early life with adult health and human capital in countries of low and middle income: findings from five birth cohort studies. Lancet 2013;382:525–34.
9. Koletzko B, von Kries R, Closa R, et al. Can infant feeding choices modulate later obesity risk? Am J Clin Nutr 2009;89 (suppl):1502S–8.
10. Dewey KG, Beaton G, Fjeld C, et al. Protein requirements of infants and children. Eur J Clin Nutr 1996;50 (suppl 1):S119–50.
11. Fomon SJ. Requirements and recommended dietary intakes of protein during infancy. Pediatr Res 1991;30:391–5.
12. Koletzko B, von Kries R, Closa R, et al. Lower protein in infant formula is associated with lower weight up to age 2 years: a randomized clinical trial. Am J Clin Nutr 2009;89:1837–45.
13. Alexy U, Kersting M, Sichert-Heller W, et al. Macronutrient intake of 3- to 36-month-old German infants and children: results of the DONALD study. Ann Nutr Metab 1999;43:14–22.
14. Hoppe C, Mølgaard C, Thomsen BL, et al. Protein intake at 9 months of age is associated with body size but not with body fat in 10-year-old Danish children. Am J Clin Nutr 2004;79:494–501.
15. Butte NF, Fox MK, Briefel RR, et al. Nutrient intakes of US infants, toddlers, and preschoolers meet or exceed dietary reference intakes. J Am Diet Assoc 2010;110:S27–37.
16. O¨ hlund I, Hernell O, Ho¨ rnell A, et al. BMI at 4 years of age is associated with previous and current protein intake and with paternal BMI. Eur J Clin Nutr 2010;64:138–45.
17. Rolland-Cachera MF, Deheeger M, Akrout M, et al. Influence of macronutrients on adiposity development: a follow up study of nutrition and growth from 10 months to 8 years of age. Int J Obes Relat Metab Disord 1995;19:573–8.
18. Gunnarsdottir I, Thorsdottir I. Relationship between growth and feeding in infancy and body mass index at the age of 6 years. Int J Obes 2003;27:1523–7.
19. Gu¨ nther ALB, Buyken AE, Kroke A. Protein intake during the period of complementary feeding and early childhood and the association with body fat at 7 years of age. Am J Clin Nutr 2007;85:1626–33.
20. Singhal A, Kennedy K, Lanigan J, et al. Nutrition in infancy and long- term risk of obesity: evidence from 2 randomized controlled trials. Am J Clin Nutr 2010;92:1133–44.
21. Arenz S, Ru¨ckerl R, Koletzko B, et al. Breastfeeding and childhood obesity: a systematic review. Int J Obes 2004;28:1247–56.
22. Owen CG, Martin RM, Whincup PH, et al. Effect of infant feeding on the risk of obesity across the life course: a quantitative review of published evidence. Pediatrics 2005;115:1367–77.
23. Harder T, Bergmann R, Kallischnigg G, et al. Duration of breastfeeding and risk of overweight: a meta-analysis. Am J Epidemiol 2005;162:397– 403.
24. Baker JL, Michaelsen KF, Rasmussen KM, et al. Maternal prepregnant body mass index, duration of breastfeeding, and timing of complementary food introduction are associated with infant weight gain. Am J Clin Nutr 2004;80:1579–88.
25. Agras WS, Hammer LD, McNicholas F, et al. Risk factors for childhood overweight: a prospective study from birth to 9.5 years. J Pediatr 2004;145:20–5.
26. Whitaker RC. Predicting preschooler obesity at birth: the role of maternal obesity in early pregnancy. Pediatrics 2004;114:e29.
27. Li C, Kaur H, Choi WS, et al. Additive interactions of maternal prepregnancy BMI and breast-feeding on childhood overweight. Obes Res 2005;13:362–71.
28. Manios Y, Moschonis G, Grammatikaki E, et al. Determinants of childhood obesity and association with maternal perceptions of their children’s weight status: the ‘‘GENESIS’’ study. J Am Diet Assoc 2010; 110:1527–31.
29. Steyn NP, Labadarios D, Nel J, et al. What is the nutritional status of children of obese mothers in South Africa? Nutrition 2011;27:904–11.
30. Hochner H, Friedlander Y, Calderon-Margalit R, et al. Associations of maternal prepregnancy body mass index and gestational weight gain with adult offspring cardiometabolic risk factors: the Jerusalem Peri-natal Family Follow-up Study. Circulation 2012;125:1381–9.
31. Yu Z, Han S, Zhu J, et al. Pre-pregnancy body mass index in relation to infant birth weight and offspring overweight/obesity: a systematic review and meta-analysis. PLOS One 2013;8:e61627.
32. Deierlein AL, Siega-Riz AM, Adair LS, et al. Effects of pre-pregnancy body mass index and gestational weight gain on infant anthropometric outcomes. J Pediatr 2011;158:221–6. 33. WHO Child Growth Standards. Growth velocity based on weight, length and head circumference. 2009. http://www.who.int/childgrowth/stan- dards/w_velocity/en. Accessed November 13, 2013.
34. Chouraqui J-P, Van Egroo L-D, Fichot M-C. Acidified milk formula supplemented with Bifidobacterium lactis: impact on infant diarrhea in residential care settings. J Pediatr Gastroenterol Nutr 2004;38:288–92.
35. Saavedra JM, Abi-Hanna A, Moore N, et al. Long-term consumption of infant formulas containing live probiotic bacteria: tolerance and safety. Am J Clin Nutr 2004;79:261–7.
36. Szajewska H. Microbiota modulation: can probiotics prevent/treat dis- ease in pediatrics? Nestle Nutr Inst Workshop Ser 2013;77:99–110.
37. Codex Alimentarius Commission. CODEX STAN 72-1981 and CO- DEX STAN 156-1987. http://www.codexalimentarius.org/standards/ list-of-standards/en/?provide=standards&orderField=fullReference& sort=asc&num1=CODEX. Accessed November 13, 2013.
38. WHO. WHO Child Growth Standards [WHO web site]. 2006. http:// www.who.int/childgrowth/en/. Accessed November 13, 2013.
39. Venables WN, Ripley BD. Modern Applied Statistics with S. 4th ed. New York: Springer; 2002.
40. Szajewska H, Chmielewska A. Growth of infants fed formula supple- mented with Bifidobacterium lactis Bb12 or Lactobacillus GG: a systematic review of randomized controlled trials. BMC Pediatr 2013; 13:185.
41. Steenhout PG, Rochat F, Hager C. The effect of Bifidobacterium lactis on the growth of infants: a pooled analysis of randomized controlled studies. Ann Nutr Metab 2009;55:334–40.
42. Braegger C, Chmielewska A, Decsi T, et al. Supplementation of infant formula with probiotics and/or prebiotics: a systematic review and comment by the ESPGHAN Committee on Nutrition. J Pediatr Gastroenterol Nutr 2011;52:238–50.
43. Socha P, Grote V, Gruszfeld D, et al. Milk protein intake, the metabolic- endocrine response, and growth in infancy: data from a randomized clinical trial. Am J Clin Nutr 2011;94 (suppl):1776S–84S.
44. Ra¨iha¨ NCR, Fazzolari-Nesci A, Cajozzo C, et al. Whey predominant, whey modified infant formula with protein/energy ratio of 1.8 g/100 kcal: adequate and safe for term infants from birth to four months. J Pediatr Gastroenterol Nutr 2002;35:275–81.
45. Ziegler EE, Jeter JM, Drulis JM, et al. Formula with reduced content of improved, partially hydrolyzed protein and probiotics: infant growth and health. Monatsschr Kinderheilk 2003;151:S65–71.
46. Haschke F, Steenhout P, Grathwohl D, et al. Evaluation of growth and early infant feeding: a challenge for scientists, industry and regulatory bodies. World Rev Nutr Diet 2013;106:33–8.
47. Ciampa PJ, Kumar D, Barkin SL, et al. Interventions aimed at decreas- ing obesity in children younger than 2 years. A systematic review. Arch Pediatr Adolesc Med 2010;164:1098–104.
48. Dattilo AM, Birch L, Krebs NF, et al. Need for early interventions in the prevention of pediatric overweight: a review and upcoming directions. J Obes 2012;2012:123023.

