Dietary strategies for early immune modulation in primary food allergy prevention
ABSTRACT
As the global incidence of food allergies continues to rise, effective primary prevention strategies remain a public health priority. Eczema in early infancy is a major risk factor for IgE-mediated food allergies. Exposure to food allergens via inflamed skin promotes a cascade of allergic immune responses and increases the risk of IgE- mediated food allergies. By contrast, early exposure to food allergens via the gut is likely to induce oral tolerance. Landmark studies have demonstrated a significant reduction in the incidence of egg and peanut allergy in response to the early dietary introduction of food allergens.
As a result, early feeding guidelines in countries with a high incidence of food allergies have shifted from recommending prolonged allergen avoidance to a policy of early introduction from around 6 months of age, as part of a diverse complementary diet. Uncertainties remain regarding the optimum timing for the introduction of specific food allergens, as well as the minimum effective frequency and dose. Exclusive breast feeding for around 6 months, and continuation to 2 years, is generally recommended but does not appear to consistently prevent food allergies. Perinatal and intermittent supplementary feeding with cow’s milk-based formula may increase the risk of cow’s milk protein allergy. Maternal food allergen avoidance during pregnancy and lactation is not effective. However, maternal consumption of food allergens during breast feeding appears to increase their preventive effect for the infant in conjunction with the early introduction of allergens into the complementary diet. Data on the dietary supplementation of pregnant women, breastfeeding mothers and infants with vitamin D, omega-3 polyunsaturated fatty acids, prebiotics or probiotics for the purpose of food allergy prevention are insufficient to make firm clinical recommendations. In infants with eczema, a combination of early allergen introduction with regular emollient treatment may have additive preventive effects, but clinical trials have so far had mixed outcomes. Further clinical trials combining dietary strategies with interventions aiming to restore the disrupted skin barrier in infants with eczema may provide new insights into effective food allergy prevention in at-risk populations.
BACKGROUND
Despite a greater understanding of the risk factors and immunological mechanisms for IgE-mediated food allergies, their incidence continues to increase in many global regions.1 2 IgE-mediated food allergies increasingly persist to adult age, and clinical patterns appear to be getting more severe. Therefore, the prevention of food allergies and other allergic disorders remains a major public health priority. Currently, prevention strategies have mainly focused on IgE- mediated food allergies as the mechanisms involved in non-IgE-mediated food allergy remain largely unknown.3 Eczema is the main known risk factor for IgE-mediated food allergies, although an increasing number of children without eczema or other identifi- able risk factors is developing food allergies.4 This highlights the need for more accurate markers of allergy risk that would allow early and targeted interventions in the prevention of food allergies and other atopic disorders.
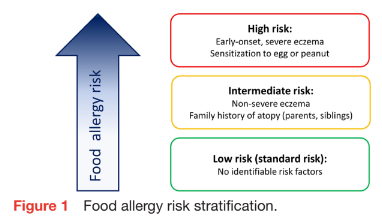
Early-onset eczema has been identified as the most significant and easily recognisable risk factor for food allergy, whereby early onset and high severity confer the highest risk.4 Two genes involved in skin barrier function (filaggrin and SPINK5) are inde- pendently associated with both eczema and food allergy.2 Apart from hereditary factors, the risk of food allergies and other allergic diseases is modulated by a complex array of environmental risk factors and early life exposures, including air pollution, maternal tobacco smoking, urban lifestyles, environ- mental greenness, airborne pollen levels and exposure to sunlight.5 An individualised risk assessment considering all genetic, environ- mental, and other risk factors is therefore not feasible. A widely accepted risk stratification of food allergy risk is shown in figure 1.
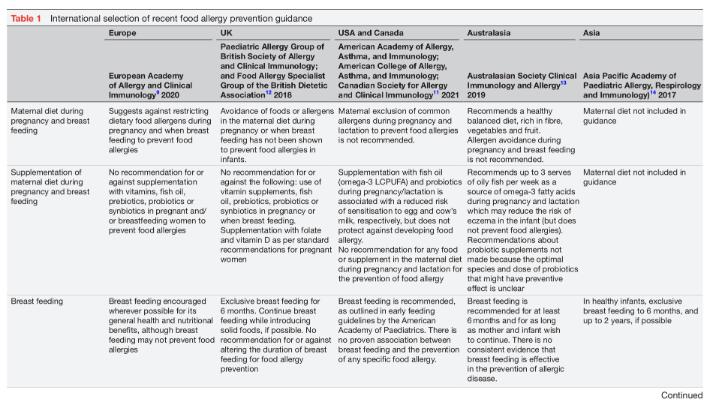
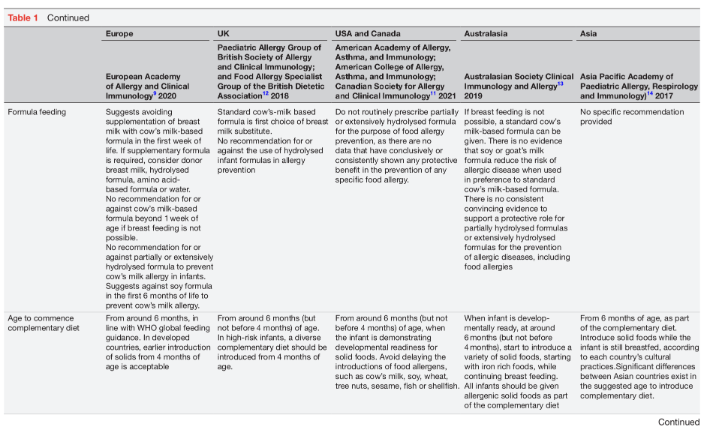
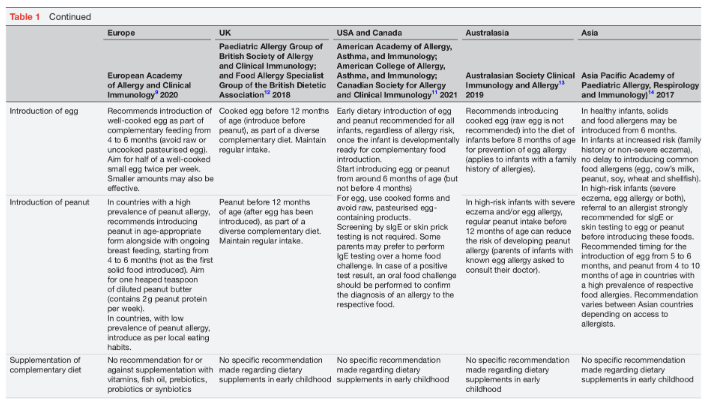
Diet is one of the few individually modifiable risk factors for food allergy. Dietary interventions, therefore, play a significant role in food allergy prevention. However, recent intervention trials suggest that diet on its own may not be sufficient to consistently prevent food allergies.6 7 Dietary food allergy prevention has three intervention windows: pregnancy, lactation and the weaning period. This review will provide an overview of the mechanisms involved in oral tolerance development, the influence of the developing microbiome and importance of epigenetic factors. Furthermore, the strategies for dietary immune modulation will be summarised, with particular reference to current international early feeding and food allergy prevention guidance (table 1).8–15
Immunological mechanisms involved in tolerance development to food proteins
Oral tolerance represents an active state of nonresponsiveness to ingested food allergens.2 3 The gut- associated lymphoid tissue plays a key role in the induction and maintenance of oral tolerance.2 In infancy, immune processing of foods in the gut promotes tolerance to dietary allergens. As a first step, degradation of intact antigens by gastric acid generates peptides of lower allergenicity. Predigested antigens pass through the gut and may cross the intestinal epithelial barrier passively between enterocytes, or via active transport by microfold cells (M cells) in the Peyer’s patches. Luminal antigens get sampled by subepithelial macrophages and CD103+ dendritic cells in the lamina propria.16 These secrete interleukin-10 (IL-10) and transforming growth factor-b (TGF-b) which induce regulatory T-cells (Treg). Retinoic acid, a vitamin A derivative, promotes the proliferation of FoxP3+ Treg in regional lymph nodes. Treg activated by CD103+ dendritic cells express specific gut-homing molecules (integrin-a4b7 and chemokine receptor-9) which programme the migration of FoxP3+ Treg to the small intestine. There, Treg suppress the proliferation of allergic effector cells, including T-helper 2 (Th2) cells, mast cells and basophils.3 In addition, regulatory B cells (Breg) in draining mesenteric lymph nodes inhibit the proliferation and activation of effector T-cells via the secretion of IL-10, IL-35 and TGF-b. Breg cells have been identified as the main source of food-specific IgG4, an IgG subclass which competes with IgE and reduces the risk of allergic reactions.3
Role of the gut microbiome in tolerance development
The early infantile microbiome composition and metabolomic profile modulate early immune development and tolerance acquisition.16 17 In line with the hygiene hypothesis, the risk of food allergies increases in response to reduced early microbial exposures and a lack of faecal microbial diversity. Several factors have been identified as determinants of early microbiome composition. Of these, breast feeding is the leading factor promoting the establishment of a healthy microbiome due to the effects of breast milk bacteria and human milk oligosaccharides (HMO).18 19 Conversely, disruption of the faecal microbiome due to factors associated with a Western lifestyle, such as low rates of exclusive breast feeding, a high prevalence of elective Caesarean sections and the overuse of perinatal antibiotics, deplete the pool of HMO-utilising bifidobacteria and allow the overgrowth of other gut bacteria, such as proteobacteria. This bifidobacteria-deficient state of microbial dysbiosis is thought to be a major predisposing factor for the development of allergic diseases.17
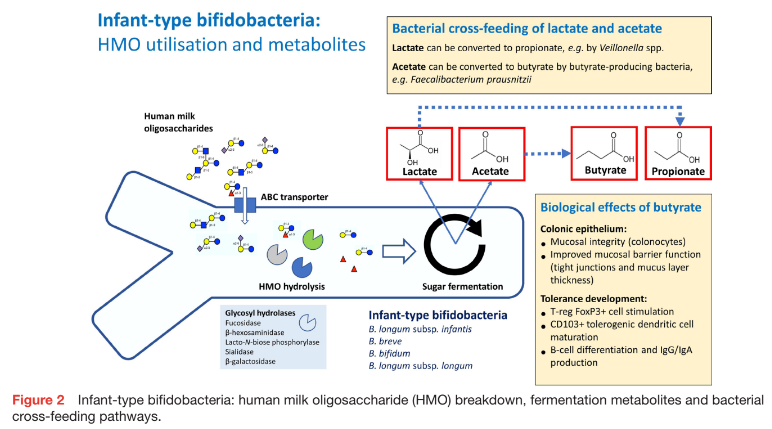
In breastfed infants, HMO support the establishment of a microbiome enriched with infant-type bifidobacteria. Bifidobacterium longum subsp. infantis is the prototype of HMO-utilising bifidobacteria which is able to assimilate HMO via specific transporters and metabolise HMO to acetate, lactate and a range of HMO breakdown prod- ucts (figure 2).17 Lactate and acetate can be metabo- lised to other short-chain fatty acids (SCFA), such as propionate and butyrate, via bacterial cross-feeding (eg, by Veillonella spp or Faecalibacterium prausnitzii).17 Faecal butyrate enhances oral tolerance via the stimulation of FoxP3+ Treg and CD103+ dendritic cells, as well as IgG and IgA production by B cells. In older infants who consume a complementary diet, fermentation of dietary fibre to butyrate promotes gut mucosal integrity and barrier function and reduces the risk of sensitisation to food allergens in the gut.17 Furthermore, infant-type bifidobacteria express aromatic lactate dehydrogenase, a recently identified enzyme which facilitates the produc- tion of aromatic lactic acids (eg, indolelactic, phenyllactic and 4-hydroxy-phenyllactic acid) from aromatic amino acids.20 Indolelactic acid is thought to modulate the immune responses of Th17-cells and monocytes via the aryl hydrocarbon and hydroxycarboxylic acid receptors.
Allergic sensitisation to food proteins
Eczema is associated with allergic skin inflammation and impaired barrier function.4 Breakdown of the cutaneous epithelial barrier and inflammation induces the expression of IL-25, IL-33 and thymic stromal lymphopoietin which promote the persistence of a Th2-skewed immune phenotype in infancy. When intact food allergens penetrate the skin barrier, Langerhans dendritic cells and other antigen presenting cells induce the generation of antigen-specific Th2 cells via the secretion of IL-4 and IL-13. These, in turn, activate B cells in the regional lymph nodes and induce the production of allergen- specific IgE (ie, sensitisation). A recently discovered subset of T follicular helper cells (Tfh13 cells) appears to be involved in the clinical expression of allergic disease after sensitisation via the induction of specific transcription factors, including IL-4 and IL-13, resulting in the production of the high-affinity IgE receptor (FceRI).21 In addition, mast cell-derived IL-4 promotes the expansion of allergen-specific Th2-skewed responses to food allergens and enhances allergic disease expression.22
Epigenetic modification of sensitisation and food allergy risk
The dramatic increase in food allergies observed over the past generation is unlikely to be due to a recent change in genomic factors. It appears more likely that environmental and dietary factors modulate gene expression via epigen- etic changes.23 Epigenetic histone modification and DNA methylation are heritable, reversible changes which affect the functionality of genetic information without changing the nucleotide sequence. A recent systematic review explored the association between epigenetic changes in candidate genes associated with food allergy.24 The review found evidence of epigenetic alteration in several gene loci involved in the maintenance of Th1/Th2 balance and Treg differentiation. These epigenetic changes may provide mechanistic insights into the pathogenesis of food allergies and may guide the development of novel diagnostic biomarkers.
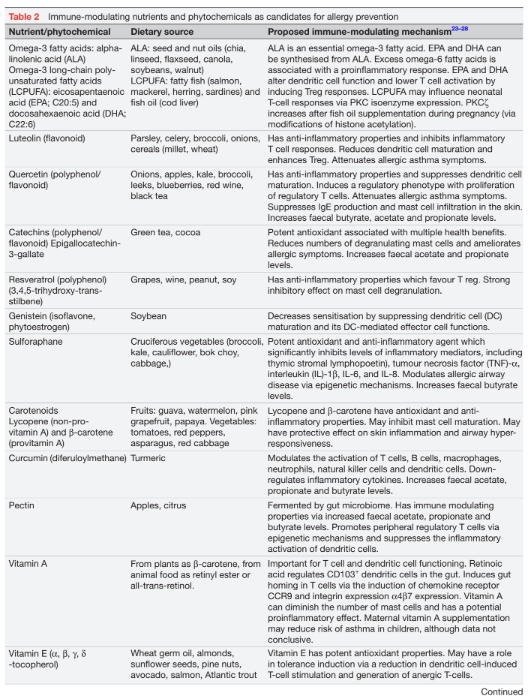
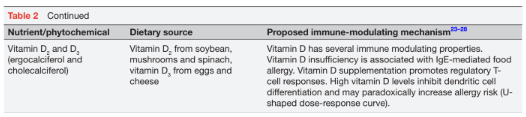
A range of early environmental exposures, including microorganisms (eg, bacteria, viruses, parasites and fungal elements) and microbial metabolites, may induce epigenetic changes that predispose to immune dysregulation and the development of food allergies. In addition, nutritional exposures may also have epigenetic effects and modulate early immune development. Examples of bioactive nutrients include breast milk, polyunsaturated fatty acids (PUFA), vitamins, as well as non-coding, plant-derived or animal-derived microRNAs (miRNA). In recent years, a range of nutrients and phytochemicals with antioxidant or anti-inflammatory properties have been explored regarding their potential to modulate or reverse immunological pathways in the development of food allergies via epigenetic changes. A list of candidate nutrients and phytochemicals is provided in table 2.
Bioactive nutrients and phytochemicals
The Mediterranean diet is high in fruits and vegetables which contain nutrients and phytochemicals with antioxidant or anti-inflammatory properties that may modulate the risk of allergies via microbiome effects and epigenetic mechanisms.23 In addition, the Mediterranean diet is high in olive oil and a range of fat-soluble nutrients, including omega-3 PUFAs, fat-soluble vitamins, flavonoids, polyphenols and carotenoids (table 2).25 26 Olive oil (oleic acid) does not appear to have significant immune modulating properties but enhances the absorption of fat-soluble bioactive nutritive compounds. Flavonoids and polyphenols with antioxidant and anti-inflammatory properties are found in vegetables, fruits, nuts and cereals.27 Other candidate nutrients with similar properties are catechin in green tea, resveratrol in grapes, sulforaphane in broccoli and genistein in soybeans. While these nutrients and phytochemicals have demonstrated immune modulating properties in vitro and in humans, their role in the prevention of food allergies remains an area of active research. Finally, fruit and vegetables are a source of fibre which is fermented in the gut to SCFA which promote tolerance development.17 The most prevalent SCFA, acetate and butyrate, are histone deacetylase (HDAC) inhibitors and are involved in epigenetic gene regulation.28 As a HDAC inhibitor, butyrate has anti-inflammatory properties and can modify gene expression and immune cell differentiation towards tolerance.
DIETARY TARGETS FOR EARLY IMMUNE MODULATION
The most promising window for immune modulation is from conception to the end of the second year of life, the first 1000 days of life, which includes dietary interventions during pregnancy and lactation, as well as the weaning period. A broad range of dietary factors have been shown to promote immune modulation and may prevent food allergies via three main pathways: (1) direct effects of nutrients on immune-competent cells involved in tolerance development, (2) epigenetic modification of allergy-specific genetic pathways and (3) modification of immune responses via the gut microbiome and associated metabolites. The following section will describe the current dietary food allergy prevention strategies in pregnant women, breastfeeding mothers and young infants receiving a complementary diet.
Maternal diet during pregnancy and lactation
The diet of pregnant women is thought to have immune modulating effects for the unborn infant via modification of the maternal microbiome and epigenetic modification of allergic pathways.23 However, data so far have not demonstrated a consistent protective effect of the maternal diet on food allergies. A Western lifestyle and diet are associated with an increased risk of food allergies. These diets significantly rely on processed foods, are often high in refined sugars and saturated fats, and are low in dietary fibre from fruit or vegetables. There is recent evidence from non-human primates that a Western-style maternal diet during pregnancy has lasting effects on proinflammatory gene expression by fetal haematopoietic stem and progenitor cells which predisposes the offspring to inflammatory disease throughout their lifespan.29 High sugar intakes during pregnancy seem to increase the risk of asthma and other allergic manifestations in childhood.30 Similarly, a maternal high-fat diet has been shown to trigger increased Th2 cytokine production by CD4+ cells via epigenetic changes which may contribute to an increased allergy risk in the offspring.31 Dietary fibre is important for maintaining a healthy maternal gut microbiome and confers tolerogenic effects via fermentation to butyrate and other SCFA.17 These considerations highlight the importance of a healthy, balanced and nutritionally complete diet as the foundation of any dietary prevention strategy in pregnant women and during breast feeding.
Maternal diets high in omega-3 fatty acids
Omega-3 fatty acids (FAs) are a family of unsaturated fatty acids with anti-inflammatory properties.32 Dietary sources of omega-3 FAs include oily fish (eg, mackerel, tuna, salmon, sardines), fish oil, seeds (eg, linseed, flaxseed, chia seed) and plant oils (eg, walnut, canola). In Western diets, consumption of omega-6 FAs usually outweighs the intake of omega-3 FAs, an imbalance which promotes a proinflammatory immune phenotype. Three omega-3 fatty long-chain polyunsaturated fatty acids (LCPUFA) have known health benefits, that is, eicosapentaenoic acid (EPA), docosapentaenoic acid and docosahexaenoic acid (DHA), including improved phospholipid membrane integrity, as well as cerebral and retinal development (table 2).32 LCPUFAs are able to regulate macrophage activation and Treg which both promote tolerance development.32 One of the mechanisms involved in the modulation of infantile T cell responses occurs via epigenetic modification of histone acetylation in the PKCx promotor gene region.33
Maternal supplementation with DHA and EPA increases their concentrations in breast milk.34 Fish oil supple- mentation (containing EPA and DHA) during pregnancy resulted in reduced cord blood concentrations of Th-2 cytokines (IL-4 and IL-13), as well as increased levels of oral tolerance-inducing TGF-b.35 High-dose fish oil supplementation in pregnant women with a family history of allergies was associated with significantly lower rates of eczema and egg sensitisation in the infant at 12 months of age.36 However, current data do not support a beneficial effect for food allergies.36,37 A systematic review concluded that despite the reduction in egg sensitisation, a consistent effect on food allergy prevention was not demonstrated.38 Recent food allergy prevention guidelines, therefore, do not specifically support omega-3 LCPUFA supplementation during pregnancy.9 11
Vitamin D supplementation during pregnancy
In its active form, vitamin D3 (calcitriol) regulates calcium and phosphate metabolism, and has effects on keratinocytes, endothelial cells, osteoblasts and lymphocytes.32 The majority of vitamin D3 is generated in the skin after UVB light exposure which induces the conversion of 7-dehydrocalciferol to provitamin D3. Dietary vitamin D is ingested in the form of Vitamin D3 from animal sources (eg, eggs, cheese), and as vitamin D2 (ergocalciferol) from vegetables and plant foods. Vitamin D is thought to promote tolerance development via the proliferation of Treg cells and an increase in anti-inflammatory cytokines, such as IL-10. In an Australian study, serum vitamin D insufficiency below 50 nmol/L was associated with a significantly increased risk of egg and/or peanut allergy.39 This finding concurred with the observation that the prevalence of food allergy and eczema was higher in regions with less sun exposure and lower skin-derived vitamin D levels.40 By contrast, excess vitamin D levels may have undesirable immune-modulating effects and increase the risk of allergic sensitisation via the inhibi- tion of dendritic cell maturation, suggesting a U-shaped effect curve.32,41 The WHO recommends a daily vitamin D intake for women of 600 IU/day. Additional supplementation during pregnancy for the purposes of food allergy prevention is currently not recommended.11 32
Probiotic supplementation during pregnancy and lactation
The effects of probiotics on the innate immune system are mediated either via specific bacterial metabolites and or via direct interaction of probiotic bacteria with Toll-like receptors (TLR), resulting in the promotion of T-helper 1 (Th1) differentiation, production of regulatory cytokines (IL-10 and TGF-b) and enhanced intestinal IgA responses.42 Several studies have demonstrated that administration of probiotics from the third pregnancy trimester, and sometimes continued during lactation, was associated with a significant reduction in atopic eczema in the offspring.43 However, results have been varied, depending on the probiotic strain, dose, timing and food matrix used. A study using Lactobacillus acidophilus even showed a paradoxical increase in allergic sensitisation.44 Despite some positive findings, the role of probiotics in primary food allergy prevention remains poorly defined.43 Strain-specific and dose-specific preventive effects need to be established in clinical trials before probiotics can be recommended for food allergy prevention.
Breast feeding
Breast milk contains a complex mixture of immunologically active ingredients, including immunoglobulins, cytokines, miRNAs and maternal micro-organisms. In addition, breast milk contains trace amounts of intact food antigens from the maternal diet. Each of these factors may contribute to early tolerance development. While exclusive breast feeding for at least 4–6 months, and further breast feeding until 2 years of age is universally recommended for all its associated nutritional and immunological benefits, breast feeding itself does not appear to provide a significant protective effect against any specific food allergy.11 This fact suggests that other genetic, dietary or environmental factors modify the impact of breast feeding on allergic disease and food allergies.45
Breast feeding is a key factor in the establishment of a faecal microbiome high in infant-type bifidobacteria.20 Microbiota in breast milk and HMO have a major influence on the early colonisation of the gut and tolerance development.46 Breast milk with a low microbial richness appears to confer reduced protection against developing allergies.47 Specific HMO-utilising bifidobacteria (B. longum subsp. infantis, B. breve, B. bifidum, B. longum subsp. longum) enhance mucosal tolerance via the effects of specific metabolites (eg, acetate, lactate, aromatic lactic acids) on Treg cell proliferation, as well as interaction with Toll-like receptors (eg, TLR2) (figure 2).48–50 As HMO concentrations and composition vary significantly between mothers depending on expression of the fucosyl- transferase-2 (FUT2) gene, secretor or non-secretor status for this enzyme may impact the allergy risk of breastfed infants. Breast milk containing FUT2-dependent HMO (from ‘secretor’ mothers) appears to decrease the risk of sensitisation in infants at increased risk of allergies.51
Recent research has focused on the role of breast milk miRNAs in the regulation of tolerance.52 Pathways significantly affected by miRNAs include TGF-b signalling, T cell receptor signalling, TLR signalling, Jak-STAT signal- ling and Th1/Th2 differentiation. The involvement of miRNAs in TGF-b secretion is of particular interest as TGF-b regulates the interaction of CD103+ dendritic cells with naïve Th cells in the mesenteric lymph node. TGF-b enhances the proliferation of FoxP3+ Treg which play a leading role in tolerance development. Differences in miRNA profiles in breast milk may further explain the diverse allergy outcomes in breastfed infants.45
Formula feeding
When breast feeding is not possible, a range of infant formulas are used. None of these are known to have a direct preventive effect on food allergies. Perinatal or intermittent feeding with cow’s milk-based formula may increase the risk of developing cow’s milk allergy, although data are not conclusive.53 A meta-analysis of partially hydrolysed formula (PHF) and extensively hydrolysed formula did not demonstrate a preventive benefit regarding food allergies or eczema.54 However, the role of PHF remains an area of debate. While several recent prevention guidelines do not recommend PHF feeding for food allergy prevention,11 13 the European Academy of Allergy and Clinical Immunology (EAACI) guidelines made no recommendation for or against PHF. A meta-analysis underpinning these recommendations was based on pooled data from a diverse range of hydro- lysed formulas with different peptide profiles and there- fore may have missed a preventive effect associated with specific PHF products.11 In a meta-analysis limited to a 100% whey-based PHF there appeared to be a preventive effect for eczema in infants with hereditary allergy risk, but no significant effect against food allergies.55 A recent randomised clinical trial of another whey-based PHF found a significantly lower incidence of eczema and suggested a preventive effect against CMPA in high- risk infants.56 However, that study was not adequately powered to adequately assess the effect of PHF on the risk of CMPA.
Prebiotic supplementation of infant formulas
Several infant formulas are supplemented with non- digestible, prebiotic oligosaccharides. These include long-chain fructo-oligosaccharides (FOS) and short-chain galacto-oligosaccharides (GOS). GOS and FOS have been shown to promote faecal bifidobacteria strains in formula-fed infants. However, this does not appear to include an enrichment of infant-type bifidobacteria which use HMO as their specific substrate.20 Few data on the role of prebiotics in the prevention of allergies are available. A study in infants fed a hydrolysed formula supplemented with FOS/GOS suggested a reduced risk of eczema.57 Based on low-level evidence, a Cochrane review in 2013 concluded that formulas supplemented with GOS/FOS reduced the risk of eczema, particularly in high-risk infants.58 Further studies on the prevention benefits of prebiotic supple- mentation in formula-fed infants are required.
Infant formulas supplemented with breast milk-identical HMO have recently become available. As described earlier, HMO promote the development of a healthy early microbiome development and have the potential for immune modulation and the prevention of allergic disease.59 Beneficial effects of formula supplementation with 2’-fucosyllactose (2’-FL) and lacto-N-neotetraose (LNnT) include an enrichment of the gut microbiome with HMO-utilising bifidobacteria, as well as a reduction in respiratory infections and antibiotic use.60 However, no data regarding the role of HMO-supplemented formulas for food allergy prevention in non-breastfed infants are yet available, and further studies are needed.
Complementary weaning diet and early allergen introduction
According to the WHO feeding guidelines, infants should commence a healthy, diverse and nutritionally complete complementary diet from around 6 months of age, while continuing to breastfeed, if possible.8 In developed countries with a high incidence of food allergies, complementary solids are often introduced from 4 months of age, including iron-containing foods, such as fortified infant cereals. A diverse complementary has been shown to be associated with a lower risk of food allergies.61
The early introduction of allergenic foods into the complementary diet has been the most significant recent innovation in strategies to prevent IgE-mediated food allergies.62 This approach is based on the dual allergen exposure hypothesis by which early gastrointestinal expo- sure to food allergens appears to protect from food allergy, whereas allergen exposure via inflamed skin in infants with eczema increases the risk of food sensitisation and allergic disease.3 Two landmark clinical trials, ‘Learning Early About Peanut Allergy’ (LEAP)63 and ‘Enquiring About Tolerance’ (EAT)64 have challenged the former practice of prolonged food allergen avoidance and instead demonstrated that the early dietary introduction of peanut and egg significantly reduced the incidence of allergies to these foods.63 64 In following years, early feeding and food allergy prevention guidelines have advocated for the early introduction of food allergens as part of the complementary diet from around 6 months, but not before 4 months of age.11–13 However, the implementation of this policy at population level has been fraught with difficulties due to a range of factors, including the lack of practical concepts of how to introduce and maintain several food allergens in the complementary diet.62 In addition, parental fear of allergic reactions in their infant may have contributed to a delayed uptake of guidelines.
The LEAP study enrolled infants with egg allergy or significant eczema and randomised them between receiving peanut from 4 months or continued strict avoidance.63 The study showed overwhelming evidence of a protective effect against peanut allergy (82% risk reduction) in those who introduced peanut early. The EAT Study prospectively examined if the early introduction of 6 food allergens (from 3 months of age while breast feeding) could reduce the risk of food allergy in a non-allergic population.64 On per-protocol analysis, there was a significant protective effect against egg and peanut allergy. However, the study failed on intention-to- treat analysis due to a considerable proportion of participants who were unable to adhere to the study regimen. This raised questions around the logistics of introducing foods early in infancy, including finding suitable food formats that would allow the delivery of food proteins in young infants. Australia, a country with one of the highest rates of food allergy in the world,65 was among the first to proactively promote the early allergen introduction in feeding and prevention guidelines.13 Disappointingly, a recent epidemiological study found that despite guide- lines recommending the early introduction of peanut since 2016, the overall incidence of peanut allergy had not shifted significantly, with the highest risk of peanut allergy seen among infants of East Asian families with a recent migration background.6 This study highlights that the prevention of food allergies remains complex and may not be achievable by stand-alone dietary interventions.5
Many open questions remain around the early intro- duction of food allergens. The optimum timing for specific food allergens is unclear. While introducing egg from 3 to 6 months was associated with a decreased risk of egg allergy, the suggested timing for the introduction of peanut appears to be between 3 and 10 months of age.62 Interestingly, an early low-dose introduction for egg seemed to confer greater protection against egg allergy.66 67 For cow’s milk, data were difficult to interpret but suggested that the early introduction from the first day of life to 4 months reduced the risk of CMPA.62 Data for the timing of other foods were insufficient to make recommendations. Other controversial issues are the minimum effective dose and frequency for the early intro- duction of specific foods. Finally, it is unclear if the early allergen introduction is recommended in countries with a low incidence of food allergies as it may be associated with an increase in food allergies from a low baseline. This is reflected in the EAACI guidelines which recommend the early introduction of peanut only in countries with a high incidence of peanut allergy.9
SUMMARY AND CONCLUSION
The maternal diet during pregnancy and lactation, as well as the early infantile diet have been identified as modifiable risk factors for food allergies. Dietary interventions aim to modulate the food allergy risk via direct effects on immune-competent cells, epigenetic modification of genes involved in the regulation of allergic pathways, and indirect effects via the gut microbiome and associated metabolites. A range of bioactive dietary compounds may be able to exert epigenetic effects on genetic pathways involved in the regulation of allergic disease.
Diets in pregnant women and breastfeeding mothers should be nutritionally complete, diverse and high in fibre from fruits, vegetables and cereal grains. Maternal allergen elimination diets during pregnancy and lactation are not recommended. To the contrary, maternal consumption of allergenic foods while breast feeding may reduce the sensitisation risk when the corresponding food is introduced as part of an early complementary diet. Data on maternal supplementation with omega-3 LCPUFA, vitamin D, probiotics or dietary fibre for the purpose of food allergy prevention are at this stage inconclusive.
Early feeding guidelines recommend exclusive breast feeding for 6 months for a multitude of associated benefits, although breast feeding on its own did not appear to significantly reduce the risk of food allergies. The early introduction of food allergens into the complementary diet from around 6 months (but not before 4 months) of age has been shown to be highly effective in reducing the incidence of egg and peanut allergy. The concept of early allergen introduction is likely to apply to other food allergens as well. However, the optimum allergen dose, intervention window and minimum frequency of dietary exposure still need to be defined. Recent studies have suggested that early and ongoing low-dose exposure may be sufficient, but further prospective studies are needed. While dietary supplementation with prebiotics, probiotics or synbiotics may be able to modify the gut micro- biome, data on their use for food allergy prevention are insufficient for clinical recommendations. Similarly, the supplementation of infants with vitamin D or LCPUFA for food allergy prevention is currently not supported. There is emerging evidence that combining topical measures to restore the skin barrier in infants with eczema with early food introduction may have a synergistic effect, but studies have had conflicting outcomes.68 Further studies on combined nutritional and dermatological interventions in the prevention of eczema and food allergies are recommended.
______________________________________________
Funding. The author has not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests. RGH is an employee of Nestlé Health Science.
Patient consent for publication. Not applicable.
Provenance and peer review. Not commissioned; externally peer reviewed.
REFERENCES
Sampath V, Abrams EM, Adlou B, et al. Food allergy across the globe. J Allergy Clin Immunol 2021;148:1347–64.
Renz H, Allen KJ, Sicherer SH, et al. Food allergy. Nat Rev Dis Primers 2018;4:17098.
Nuyttens L, De Vlieger L, Diels M, et al. The clinical and immunological basis of early food introduction in food allergy prevention. Front Allergy 2023;4:1111687.
Martin PE, Eckert JK, Koplin JJ, et al. Which infants with eczema are at risk of food allergy? results from a population-based cohort. Clin Exp Allergy 2015;45:255–64.
Peters RL, Mavoa S, Koplin JJ. An overview of environmental risk factors for food allergy. Int J Environ Res Public Health 2022;19:722.
Soriano VX, Peters RL, Moreno-Betancur M, et al. Association between earlier introduction of peanut and prevalence of peanut allergy in infants in Australia. JAMA 2022;328:48–56.
Vandenplas Y, Meyer R, Chouraqui J-P, et al. The role of milk feeds and other dietary supplementary interventions in preventing allergic disease in infants: fact or fiction? Clin Nutr 2021;40:358–71.
World Health Organization. Factsheet: infant and young child feeding. 2021. Available: https://wwwwhoint/news-room/fact-sheets/detail/infant-and-young-child-feeding;accessed [Accessed 26 May 2023].
Halken S, Muraro A, de Silva D, et al. EAACI guideline: preventing the development of food allergy in infants and young children (2020 update). Pediatr Allergy Immunol 2021;32:843–58.
Togias A, Cooper SF, Acebal ML, et al. Addendum guidelines for the prevention of peanut allergy in the United States: summary of the national Institute of allergy and infectious diseases-sponsored expert panel. J Acad Nutr Diet 2017;117:788–93.
Fleischer DM, Chan ES, Venter C, et al. A consensus approach to the primary prevention of food allergy through nutrition: guidance from the American academy of allergy, asthma, and immunology; American college of allergy, asthma, and immunology; and the Canadian society for allergy and clinical immunology. J Allergy Clin Immunol Pract 2021;9:22–43.
Turner PJ, Feeney M, Meyer R, et al. Implementing primary prevention of food allergy in infants: new BSACI guidance published. Clin Exp Allergy 2018;48:912–5.
Joshi PA, Smith J, Vale S, et al. The Australasian society of clinical immunology and allergy infant feeding for allergy prevention guidelines. Med J Aust 2019;210:89–93.
Tham EH, Shek L-C, Van Bever HP, et al. Early introduction of allergenic foods for the prevention of food allergy from an Asian perspective-an Asia Pacific association of pediatric allergy, respirology & immunology (APAPARI) consensus statement. Pediatr Allergy Immunol 2018;29:18–27.
Ebisawa M, Ito K, Fujisawa T. Committee for Japanese pediatric guideline for food allergy, the Japanese society of pediatric allergy and clinical immunology, Japanese society of allergology. Japanese guidelines for food allergy 2020. Allergol Int 2020;69:370–86.
Tordesillas L, Berin MC. Mechanisms of oral tolerance. Clin Rev Allergy Immunol 2018;55:107–17.
Milani C, Duranti S, Bottacini F, et al. The first microbial colonizers of the human gut: composition, activities, and health implications of the infant gut microbiota. Microbiol Mol Biol Rev 2017;81:e00036-17.
Rahman T, Sarwar PF, Potter C, et al. Role of human milk oligosaccharide metabolizing bacteria in the development of atopic dermatitis/eczema. Front Pediatr 2023;11:1090048.
Stewart CJ, Ajami NJ, O’Brien JL, et al. Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature 2018;562:583–8.
Laursen MF, Sakanaka M, von Burg N, et al. Bifidobacterium species associated with breastfeeding produce aromatic lactic acids in the infant gut. Nat Microbiol 2021;6:1367–82.
Gowthaman U, Chen JS, Zhang B, et al. Identification of a T follicular helper cell subset that drives anaphylactic IGE. Science 2019;365:eaaw6433.
El Ansari YS, Kanagaratham C, Oettgen HC. Mast cells as regulators of adaptive immune responses in food allergy. Yale J Biol Med 2020;93:711–8.
Acevedo N, Alashkar Alhamwe B, Caraballo L, et al. Perinatal and early-life nutrition, epigenetics, and allergy. Nutrients 2021;13:724.
Safar R, Oussalah A, Mayorga L, et al. Epigenome alterations in food allergy: a systematic review of candidate gene and epigenome-wide association studies. Clin Exp Allergy 2023;53:259–75.
Hogenkamp A, Ehlers A, Garssen J, et al. Allergy modulation by N-3 long chain polyunsaturated fatty acids and fat soluble nutrients of the mediterranean diet. Front Pharmacol 2020;11:1244.
Singh A, Holvoet S, Mercenier A. Dietary polyphenols in the prevention and treatment of allergic diseases. Clin Exp Allergy 2011;41:1346–59.
Rakha A, Umar N, Rabail R, et al. Anti-inflammatory and anti-allergic potential of dietary flavonoids: a review. Biomed Pharmacother 2022;156:113945.
Cañas JA, Núñez R, Cruz-Amaya A, et al. Epigenetics in food allergy and immunomodulation. Nutrients 2021;13:4345.
Nash MJ, Dobrinskikh E, Soderborg TK, et al. Maternal diet alters long-term innate immune cell memory in fetal and juvenile hematopoietic stem and progenitor cells in nonhuman primate offspring. Cell Rep 2023;42:112393.
Gupta A, Singh A, Fernando RL, et al. The association between sugar intake during pregnancy and allergies in offspring: a systematic review and a meta-analysis of cohort studies. Nutr Rev 2022;80:904–18.
Lin H, Zhao Y, Zhu Y, et al. Maternal high-fat diet aggravates allergic asthma in offspring via modulating Cd4+ T-cell differentiation. Nutrients 2022;14:2508.
Feketea G, Kostara M, Bumbacea RS, et al. Vitamin D and omega-3 (fatty acid) supplementation in pregnancy for the primary prevention of food allergy in children-literature review. Children (Basel) 2023;10:468.
Harb H, Irvine J, Amarasekera M, et al. The role of PKCx in cord blood T-cell maturation towards Th1 cytokine profile and its epigenetic regulation by fish oil. Biosci Rep 2017;37:BSR20160485.
Dunstan JA, Mitoulas LR, Dixon G, et al. The effects of fish oil supplementation in pregnancy on breast milk fatty acid composition over the course of lactation: a randomized controlled trial. Pediatr Res 2007;62:689–94.
Krauss-Etschmann S, Hartl D, Rzehak P, et al. Decreased cord blood IL-4, IL-13, and Ccr4 and increased TGF-beta levels after fish oil supplementation of pregnant women. J Allergy Clin Immunol 2008;121:464–70.
Palmer DJ, Sullivan T, Gold MS, et al. Effect of N-3 long chain polyunsaturated fatty acid supplementation in pregnancy on infants' allergies in first year of life: randomised controlled trial. BMJ 2012;344:e184.
Boyle RJ, Leonardi-Bee J, Bath-Hextall FJ, et al. Probiotics for the treatment or prevention of eczema. J Allergy Clin Immunol 2009;123:266–7.
Best KP, Gold M, Kennedy D, et al. Omega-3 long-chain PUFA intake during pregnancy and allergic disease outcomes in the offspring: a systematic review and meta-analysis of observational studies and randomized controlled trials. Am J Clin Nutr 2016;103:128–43.
Allen KJ, Koplin JJ, Ponsonby A-L, et al. Vitamin D insufficiency is associated with challenge-proven food allergy in infants. J Allergy Clin Immunol 2013;131:1109–16.
Osborne NJ, Ukoumunne OC, Wake M, et al. Prevalence of eczema and food allergy is associated with latitude in Australia. J Allergy Clin Immunol 2012;129:865–7.
Yepes-Nuñez JJ, Brożek JL, Fiocchi A, et al. Vitamin D supplementation in primary allergy prevention: systematic review of randomized and non-randomized studies. Allergy 2018;73:37–49.
Rautava S, Collado MC, Salminen S, et al. Probiotics modulate host-microbe interaction in the placenta and fetal gut: a randomized, double-blind, placebo-controlled trial. Neonatology 2012;102:178–84.
Fiocchi A, Burks W, Bahna SL, et al. Clinical use of probiotics in pediatric allergy (CUPPA): a world allergy organization position paper. World Allergy Organ J 2012;5:148–67.
Taylor AL, Dunstan JA, Prescott SL. Probiotic supplementation for the first 6 months of life fails to reduce the risk of Atopic dermatitis and increases the risk of allergen sensitization in high-risk children: a randomized controlled trial. J Allergy Clin Immunol 2007;119:184–91.
Danielewicz H. Breastfeeding and allergy effect modified by genetic, environmental, dietary, and immunological factors. Nutrients 2022;14:3011.
Davis EC, Castagna VP, Sela DA, et al. Gut microbiome and breast- feeding: implications for early immune development. J Allergy Clin Immunol 2022;150:523–34.
Dzidic M, Mira A, Artacho A, et al. Allergy development is associated with consumption of breastmilk with a reduced microbial richness in the first month of life. Pediatr Allergy Immunol 2020;31:250–7.
López P, González-Rodríguez I, Sánchez B, et al. Interaction of bifidobacterium bifidum LMG13195 with HT29 cells influences regulatory-T-cell-associated chemokine receptor expression. Appl Environ Microbiol 2012;78:2850–7.
Barile D, Rastall RA. Human milk and related oligosaccharides as prebiotics. Curr Opin Biotechnol 2013;24:214–9.
Seppo AE, Autran CA, Bode L, et al. Human milk oligosaccharides and development of cow’s milk allergy in infants. J Allergy Clin Immunol 2017;139:708–11.
Sprenger N, Odenwald H, Kukkonen AK, et al. FUT2-dependent breast milk oligosaccharides and allergy at 2 and 5 years of age in infants with high hereditary allergy risk. Eur J Nutr 2017;56:1293–301.
Ahlberg E, Al-Kaabawi A, Thune R, et al. Breast milk microRNAs: potential players in oral tolerance development. Front Immunol 2023;14:1154211.
Abrams EM, Shaker MS, Chan ES, et al. Prevention of food allergy in infancy: the role of maternal interventions and exposures during pregnancy and lactation. Lancet Child Adolesc Health 2023;7:358–66.
Boyle RJ, Ierodiakonou D, Khan T, et al. Hydrolysed formula and risk of allergic or autoimmune disease: systematic review and meta- analysis. BMJ 2016;352:i974.
Szajewska H, Horvath A. A partially hydrolyzed 100% Whey formula and the risk of eczema and any allergy: an updated meta-analysis. World Allergy Organ J 2017;10:27.
Nicolaou N, Pancheva R, Karaglani E, et al. The risk reduction effect of a nutritional intervention with a partially hydrolyzed whey-based formula on cow's milk protein allergy and atopic dermatitis in high- risk infants within the first 6 months of life: the allergy reduction trial (A.R.T.), a multicenter double-blinded randomized controlled study. Front Nutr 2022;9:863599.
Moro G, Arslanoglu S, Stahl B, et al. A mixture of prebiotic oligosaccharides reduces the incidence of atopic dermatitis during the first six months of age. Arch Dis Child 2006;91:814–9.
Osborn DA, Sinn JK. Probiotics in infants for prevention of allergic disease and food hypersensitivity. Cochrane Database Syst Rev 2007:CD006475.
Zuurveld M, van Witzenburg NP, Garssen J, et al. Immunomodulation by human milk oligosaccharides: the potential role in prevention of allergic diseases. Front Immunol 2020;11:801.
Puccio G, Alliet P, Cajozzo C, et al. Effects of infant formula with human milk oligosaccharides on growth and morbidity: a randomized multicenter trial. J Pediatr Gastroenterol Nutr 2017;64:624–31.
Venter C, Maslin K, Holloway JW, et al. Different measures of diet diversity during infancy and the association with childhood food allergy in a UK birth cohort study. J Allergy Clin Immunol Pract 2020;8:2017–26.
Scarpone R, Kimkool P, Ierodiakonou D, et al. Timing of allergenic food introduction and risk of immunoglobulin E-mediated food allergy: a systematic review and meta-analysis. JAMA Pediatr 2023;177:489–97.
Du Toit G, Roberts G, Sayre PH, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med 2015;372:803–13.
Perkin MR, Logan K, Tseng A, et al. Randomized trial of introduction of allergenic foods in breast-fed infants. N Engl J Med 2016;374:1733–43.
Osborne NJ, Koplin JJ, Martin PE, et al. Prevalence of challenge- proven IGE-mediated food allergy using population-based sampling and predetermined challenge criteria in infants. J Allergy Clin Immunol 2011;127:668–76.
Natsume O, Kabashima S, Nakazato J, et al. Two-step egg introduction for prevention of egg allergy in high-risk infants with eczema (PETIT): a randomised, double-blind, placebo-controlled trial. Lancet 2017;389:276–86.
Nishimura T, Fukazawa M, Fukuoka K, et al. Early introduction of very small amounts of multiple foods to infants: a randomized trial. Allergol Int 2022;71:345–53.
Yamamoto-Hanada K, Kobayashi T, Mikami M, et al. Enhanced early skin treatment for atopic dermatitis in infants reduces food allergy J Allergy Clin Immunol 2023;152:126–35.
