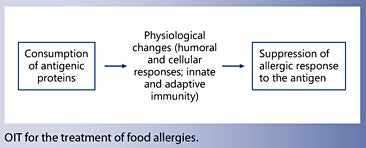
Oral Immunotherapy for Food Allergies
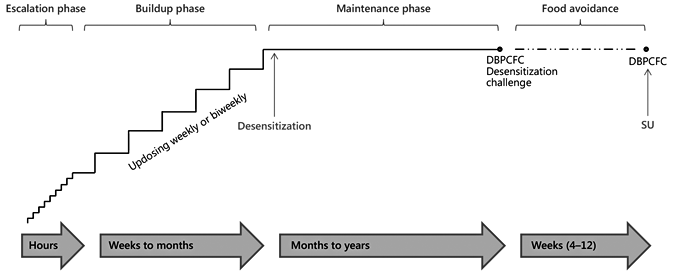
- Oral immunotherapy is an investigational therapy for food allergy, which has demonstrated efficacy in desensitizing subjects to offending food proteins.
- As most subjects regain sensitivity within days to weeks of avoidance, it is as yet unclear whether oral immunotherapy has the potential to induce permanent tolerance.
- The safety and tolerability continue to limit the utility of oral immunotherapy in routine clinical practice.
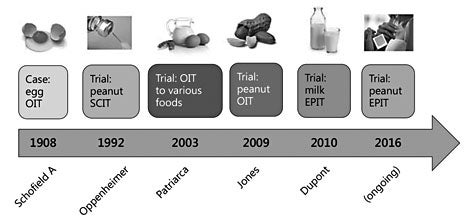
The concept of OIT for food allergy dates back to 1908, when Schofield successfully desensitized a boy with anaphylactic egg allergy. In response to the increasing prevalence of food allergies, interest in food-specific immunotherapies arose in the 1990s (fig. 1). Desensitization to foods via the subcutaneous route fell out of favor when a clinical trial with injection peanut immunotherapy reported unacceptably high rates of anaphylaxis [6]. In 2006, a successful desensitization to peanut using OIT was reported in 2 cases [7, 8]. The first clinical trial of peanut OIT, published in 2009, showed that OIT could be successfully used to induce desensitization in peanut-allergic patients with a favorable side-effect profile and low rates of anaphylaxis [9] . Sublingual immunotherapy (SLIT) for milk and peanut is less efficacious than OIT but has a favorable safety profile. Patch immunotherapy is undergoing clinical trials for milk and peanut allergy in children and adults.
While inducible T-regulatory cells (Tregs) are thought to play a central role in oral tolerance to ingested antigens [16–18], evidence supporting the role of Tregs in OIT is controversial. In mouse models of food allergy, in conjunction with improved tolerance of the food, OIT resulted in an increase in CD4+CD25+FoxP3+ cells and IL- 10- and TGF-β-producing Tregs in the lamina propria [19]. The increase in Tregs with OIT may be attributed to a replacement with polyclonal T cells, rather than to a ‘reeducation’ of existing T cells [20].
Recent studies highlight alterations in both humoral and cellular responses as well as innate and adaptive immunity. Expansion and affinity maturation of allergen-specific memory B cells during OIT suggest a potential role of these cells in tolerance acquisition [21]. IgG antibodies induced during OIT can act through an inhibitory FcγRIIb to suppress IgE-mediated hypersensitivity [22]. Dendritic cells were found to secrete increased IL-10 and IFN-α and decreased IL-6 with OIT [23]. Similar findings were observed in a cohort of children undergoing egg OIT; in addition to significant increases in IL-10 production, investigators found trends towards lower IL-5 and IL-13 production and higher tumor necrosis factor-α and interferon-γ levels [24].
Peanut
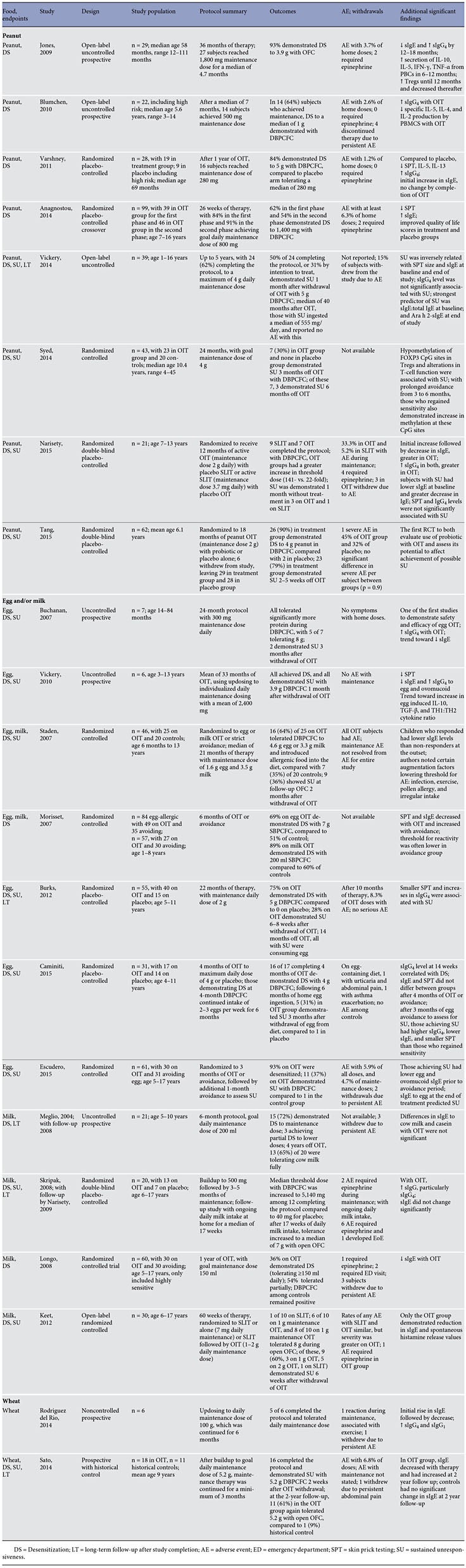
The first prospective open-label uncontrolled study reported successful desensitization in 93% of 29 participants treated with peanut OIT and a reassuring safety profile, and provided evidence of immunomodulation [9]. Another prospective cohort study, without a control group, demonstrated desensitization in 64% of the 22 subjects. Despite including high-risk patients who had had severe reactions to peanut and asthma, OIT was fairly well tolerated, with just 4 patients withdrawing due to persistent adverse events [25].
In the first randomized placebo-controlled trial (RCT) of peanut OIT with double-blinding, after 1 year of OIT, 84% of 19 subjects in the treatment group reached the goal maintenance dose and demonstrated desensitization with double-blind placebo-controlled food challenge (DBPCFC). In this cohort, which included higher-risk subjects, just 1.2% of buildup doses required treatment. Injectable epinephrine was not required in the OIT group throughout the trial, though 3 of 19 subjects did withdraw due to adverse effects [26]. In another crossover RCT using peanut OIT, 62% of 39 patients in the first-phase treatment group and 54% of 46 patients in the secondphase treatment group demonstrated desensitization to 1,400 mg of peanut during DBPCFC, compared to 0 in the placebo group. Regarding safety, the authors did not report any serious adverse events [27].
The first study to evaluate the potential for OIT to induce sustained unresponsiveness (SU) reported that, of 24 subjects who completed 5 years of peanut OIT, 50% tolerated 5 g DBPCFC after suspending OIT for 1 month and successfully added peanut to the diet [10].
In another study, of 23 OIT subjects, 7 (30%) demonstrated SU with DBPCFC 3 months after OIT discontinuation [12]. T-cell function and methylation of FOXP3 CpG sites in antigen-induced Tregs differed significantly between those demonstrating SU and those who regained sensitivity. The 7 subjects with SU continued an additional 3 months without OIT; 3 demonstrated persistent SU, while the other 4 regained sensitivity as well as increased methylation of the FOXP3 CpG sites in antigen- induced Tregs [12].
There has been one study comparing OIT with SLIT for peanut allergy. In this double-blind study, 21 subjects were randomized to active SLIT with placebo OIT or active OIT with placebo SLIT. The 16 subjects (9 SLIT and 7 OIT) who continued treatment for 12 months underwent DBPCFC, during which all subjects had a greater than 10-fold increase in challenge threshold, compared to oral food challenge (OFC) at enrollment, with a significantly higher threshold dose in the OIT group. Four weeks after withdrawal of therapy, 3 of 7 subjects on OIT and 1 of 9 subjects on SLIT demonstrated SU. Adverse reactions were generally mild but accounted for early withdrawal in 3 OIT subjects [28].
In an effort to improve the likelihood of achieving SU with OIT, a probiotic was combined with OIT. This double- blind placebo-controlled trial randomized 62 peanutallergic children to 18 months of treatment with peanut OIT given with the probiotic ( Lactobacillus rhamnosus , LGG) or placebo alone. After completion of the protocol, the subjects avoided peanut for a 2- to 5-week period, after which 23 (82%) of 29 subjects in the active treatment group demonstrated SU, compared to 1 of 28 in the placebo group. While it may have been more informative to compare peanut OIT with and without a probiotic, the results of the study suggest that the addition of a probiotic may enhance immune tolerance in OIT. Interestingly, adverse events were not significantly different between the treatment and placebo groups [29].
Egg
The first studies on egg OIT conducted by Patriarca and colleagues [30, 31] reported successful desensitization in 5 of 5 egg-allergic subjects and in 11 of 15 egg-allergic subjects in 1998 and 2003, respectively. In an uncontrolled study, after 24 months of egg OIT, all 7 eggallergic subjects tolerated significantly more egg during DBPCFC. Following a subsequent 3-month period of strict avoidance, 2 subjects demonstrated SU. In this group of 7 egg-allergic patients, reactions during the protocol were limited to the escalation and buildup phase, and none required the use of injectable epinephrine [32].
In the first RCT of egg OIT, 45 children with allergy to egg or milk were randomized to OIT or strict avoidance. After a median of 21 months of therapy, 16 (64%) of 25 children on OIT demonstrated desensitization with DBPCFC and were able to introduce previously allergenic foods into their diet, compared to 7 of 20 (35%) controls [34]. A larger study by Morisset et al. [35], which included 84 egg-allergic children (aged 1–8 years) randomized to OIT or avoidance, showed similar rates of desensitization. After 6 months of therapy, 69% of 49 children in the egg OIT group demonstrated desensitization during single-blind placebo-controlled food challenge (SBPCFC) versus 51.4% of the 35 children in the avoidance group [35].
In an effort to improve the likelihood of inducing SU, a protocol with higher, individualized doses and longer duration was utilized. After a 1-month period of strict avoidance following OIT, all 6 patients demonstrated SU with DBPCFC [33].
A randomized placebo-controlled multicenter study provided a more rigorous assessment of SU and longterm outcomes with egg OIT. With DBPCFC after 22 months of OIT, 75% of 20 subjects on OIT were desensitized, compared to 0 in the placebo group. Following a 6- to 8-week period of egg avoidance, just 28% of those in the OIT group demonstrated SU. At 36 months, all children who had demonstrated SU were consuming egg. Smaller skin tests and higher egg-specific IgG 4 were associated with an increased likelihood of achieving SU [11].
Two recent studies explored SU after a much shorter OIT period. In the RCT employing just 4 months of egg OIT followed by 6 months of ingestion of 2–3 eggs per week, 5 (31%) of 17 patients treated with egg OIT demonstrated SU after 3 months of egg avoidance, compared to just 1 patient in the placebo group [36]. Similarly, after 3 months of egg OIT followed by 1 month of avoidance, DBPCFC demonstrated SU in 11 (37%) of the 30 children on OIT, compared to 1 amongst the 31 controls [37].
Milk
Similar to egg OIT, Patriarca et al. [31] were also among the first to conduct controlled trials on milk OIT [31]. A prospective study without a control group published in 2004, reported desensitization in 15 (72%) of the 21 children completing the 6-month protocol, with 3 achieving partial desensitization. The remaining 3 could not complete the protocol due to persistent adverse effects at low doses of cow milk [38]. In a follow-up study 4 years later, the authors reported that 13 of 20 (65%) children were tolerating cow milk fully [39].
In 2007, another RCT reported similar rates of desensitization utilizing a median OIT duration of 21 months, with 16 (64%) of 25 milk-allergic children achieving desensitization in the treatment group, compared to 7 (35%) of 20 in the avoidance group [34]. In a comparatively large randomized study, Morisset et al. [35] reported the outcomes of their 6-month OIT protocol: 89% of the treatment group demonstrated successful desensitization, compared to 60% of the avoidance group.
Results of the first double-blind placebo-controlled study of milk OIT were published in 2008. Using a protocol which called for 3–4 months of maintenance following the buildup phase, the authors reported that the median threshold dose with DBPCFC was increased to 5,140 mg among the 12 patients who completed the protocol, which was significantly higher than the 40-mg threshold dose at baseline in the placebo group. Reactions occurred with a median of 35% of doses per participant [40]. With ongoing daily milk intake at home for a median of 17 weeks, a follow-up study showed increased tolerance to a median of 7 g with open OFC, with 33% of participants tolerating 16 g. The authors did report reactions with ongoing milk intake which were mostly mild; notably, injectable epinephrine was required on 6 occasions (0.2% of doses), and 1 subject developed symptoms of eosinophilic esophagitis (EoE) [41].
A RCT in a larger milk-allergic group aimed to specifically assess the efficacy of OIT among children with a history of severe reactions to milk. After 1 year of milk OIT, 11 (36%) children in the OIT group were desensitized, 16 (54%) were partially desensitized (5–150 ml), and 3 (10%) were unable to continue with therapy due to persistent abdominal or respiratory complaints. Among controls, DBPCFC after 1 year of avoidance were all positive [42].
Additional RCTs supported the efficacy and safety of milk OIT, reporting desensitization rates of approximately 90% [43, 44]. In one RCT of milk OIT, twice weekly dosing was as effective as maintenance therapy as daily dosing [45].
Milk OIT was compared to SLIT in an open-label RCT, showing improved desensitization but a worse safety proprofile than SLIT. OIT was also more effective in inducing SU (8 of 20 subjects on OIT vs. 1 of 10 subjects on SLIT) [46].
Wheat
Wheat has been the subject of few OIT trials. In a small study, 5 of 6 children successfully completed buildup and 6-month maintenance. One subject experienced symptoms during maintenance: when exercising immediately after wheat ingestion, this subject developed urticaria, which improved with antihistamine and oral steroids [47].
Using a larger cohort with a historical control group, Sato et al. [48] reported that 16 of 18 subjects achieved the target maintenance dose of 5.2 g wheat protein and, following a 2-week period of avoidance, ingested this dose without symptoms during DBPCFC. At the 2-year follow- up, 11 (61%) subjects in the treatment group tolerated 5.2 g of wheat with open OFC, compared to 1 (9%) of 11 historical controls. Regarding safety, 6.8% of the 5,778 total treatment doses resulted in symptoms, with administration of epinephrine on one occasion [48].
Regular ingestion of heated milk and egg shortens the time to tolerance acquisition to unheated forms
Baked milk diet was tested as immunotherapy for 15 highly milk-allergic patients (aged 4–12 years) who were previously unable to complete a milk OIT protocol due to severity and frequency of allergic reactions. In this uncontrolled open trial, a dose of baked milk smaller than the eliciting dose at entry OFC was gradually increased to a maintenance dose of 1.3 g per day. OFCs were performed at 6 and 12 months of treatment. Eight patients could not continue with the protocol due to IgE-mediated reactions, with a number of participants having reactions to doses that they had previously tolerated for more than 1 month. Only 3 of the 14 patients who continued with the baked milk protocol reached maintenance dosing within 12 months. For the 6 participants who continued with the protocol, there was a significant increase in challenge threshold to unheated milk. The results of this study suggest that the use of baked milk as OIT may be useful for increased threshold reactivity; however, for patients who are highly sensitive to milk, it may be less realistic as a method of attaining clinical tolerance to milk [55].
With these data, experts have recommended that for children already tolerating baked products, it is safe to continue regular ingestion at home. For those who have been strictly avoiding milk, a physician-supervised food challenge to milk will be necessary to determine whether it is safe to introduce baked products into the diet [56].
Another uncontrolled pilot study investigated the use of omalizumab in a peanut OIT protocol. The study specifically enrolled highly peanut-allergic children who are thus at a greater risk for adverse reactions with OIT. After pretreatment with omalizumab for 12 weeks, all 13 subjects (aged 8–16 years) tolerated the initial dose escalation to 500 mg peanut flour. With another 8 weeks of therapy, 12 subjects reached the goal maintenance dosing of 4,000 mg peanut flour daily, and subsequently tolerated 8,000 mg peanut during DBPCFC, at which point omalizumab was discontinued. The maintenance dose was continued at home for another 6 months without omalizumab. One subject withdrew due to persistent symptoms with OIT doses. While reactions occurred with 2% of doses, and were mostly mild, there were 5 moderate reactions and 2 severe reactions (with epinephrine required on 5 occasions), which occurred with home maintenance dosing. Only 6 of the 13 subjects had only mild or no allergic reactions. These results suggest that omalizumab facilitates rapid oral desensitization even among subjects highly sensitive to a food allergen. However, adverse reactions may continue to occur during maintenance and with home dosing following discontinuation of omalizumab [58].
The only randomized controlled study on the use of omalizumab in milk OIT used omalizumab for the pretreatment, buildup, as well as maintenance phases. The investigators randomized 57 milk-allergic subjects (aged 7–32 years) 1:1 to receive omalizumab (for 4 months before treatment and continued dosing for 24 months of OIT) or placebo. Twenty-six in each group reached maintenance dosing (3.8 g daily) 6 months into OIT. Participants underwent 10 g DBPCFC after 24 months of OIT and again after a 4-month period of milk avoidance. Though rates of desensitization (89 and 71%) and SU (48 and 36%) were not significantly different between the omalizumab and placebo groups, omalizumab did improve the safety and tolerability of OIT. The portions of doses provoking symptoms (2.1 vs. 16.1%, p = 0.005) and requiring treatment (0.0 vs. 3.8%, p = 0.0008) were significantly reduced with omalizumab [59].
The use of omalizumab was studied in a multi-food OIT protocol, which specifically enrolled children highly sensitive to the offending foods [61] . In an uncontrolled pilot study, 25 participants (median age, 7 years), all of whom failed DBPCFC to ≤ 100 mg of the offending allergens, received 8 weeks of pretreatment with omalizumab, followed by an initial dose escalation and buildup, achieving the goal maintenance dose of 4,000 mg protein per allergen in a median of 18 weeks. Omalizumab was discontinued 8 weeks into the maintenance phase. Mild reactions were reported in 5.3% of home doses, and one severe reaction (towards the beginning of the maintenance phase) was reported over the course of the study. Six months into OIT, reaction rates dropped by 70% [61] . Findings of these studies suggest that multi-food OIT may be a safe and effective approach to OIT that deserves further investigation.
The safety and tolerability of OIT continue to limit its use in routine clinical practice.
Certain augmentation factors can lower the threshold for reaction to OIT doses [34]. Five patterns associated with increased likelihood of adverse reactions during their peanut OIT trial have been identified: concurrent illness or menses, poorly controlled asthma, administration on an empty stomach, and physical exertion following a dose [64]. With the aim to enhance safety of OIT, more recent studies have put in place protocols to address these augmentation factors, with reduction in home dosing when a subject has signs of infection, and exercise avoidance in the hours immediately following a dose [47, 57].
While severe reactions account for withdrawal from OIT for a small portion of patients, the majority of patients who discontinue OIT do so because of chronic symptoms, especially chronic abdominal pain. It is unclear what portion of the patients develops symptoms due to undiagnosed EoE. A meta-analysis reported that EoE may develop in up to 2.7% of subjects on OIT to milk, peanut, egg, or wheat; however, this number may be falsely elevated due to publication bias [65]. Further studies should clarify the true incidence and elucidate whether OIT may incite EoE or unveil a pre-existing disease.
A 7-year follow-up study included 28 children (aged 6–15 years) with cow milk allergy who were enrolled in a randomized double-blind placebo-controlled milk OIT protocol [44]. In the initial study, authors reported that 24 of the 28 children were consuming milk daily at 36 months from initiation of OIT. Of the 24 participants responding to a questionnaire 7 years after initiation of milk OIT, 8 (33%) had discontinued milk altogether, 2 consumed limited amounts, and 14 (58%) reported daily consumption of milk, with 3 among these reporting symptoms associated with milk ingestion [68]. The mixed results of these follow-up studies point to the necessity of further study to improve the safety and efficacy of OIT.
Ingestion of heated milk and egg by milk- and egg-allergic children as OIT to hasten tolerance to the unheated allergen appears to be a safe and effective therapy
The writing of this article was supported by Nestlé Nutrition Institute.
- Nwaru BI, et al: Prevalence of common food allergies in Europe: a systematic review and meta-analysis. Allergy 2014; 69: 992–1007.
- Sicherer SH, Wood RA: Advances in diagnosing peanut allergy. J Allergy Clin Immunol Pract 2013; 1: 1–13; quiz 14.
- Lieberman JA, Sicherer SH: Quality of life in food allergy. Curr Opin Allergy Clin Immunol 2011; 11: 236–242.
- Versluis A, et al: Frequency, severity and causes of unexpected allergic reactions to food: a systematic literature review. Clin Exp Allergy 2015; 45: 347–367.
- Nowak-Wegrzyn A, Albin S: Oral immunotherapy for food allergy: mechanisms and role in management. Clin Exp Allergy 2015; 45: 368–383.
- Oppenheimer JJ, et al: Treatment of peanut allergy with rush immunotherapy. J Allergy Clin Immunol 1992; 90: 256–262.
- Patriarca G, et al: Oral rush desensitization in peanut allergy: a case report. Dig Dis Sci 2006; 51: 471–473.
- Mansfield L: Successful oral desensitization for systemic peanut allergy. Ann Allergy Asthma Immunol 2006; 97: 266–267.
- Jones SM, et al: Clinical efficacy and immune regulation with peanut oral immunotherapy. J Allergy Clin Immunol 2009; 124: 292–300, 300 e1–e97.
- Vickery BP, et al: SUSU to peanut in subjects who have completed peanut oral immunotherapy. J Allergy Clin Immunol 2014; 133: 468–475.
- Burks AW, et al: Oral immunotherapy for treatment of egg allergy in children. N Engl J Med 2012; 367: 233–243.
- Syed A, et al: Peanut oral immunotherapy results in increased antigen-induced regulatory T-cell function and hypomethylation of forkhead box protein 3 (FOXP3). J Allergy Clin Immunol 2014; 133: 500–510.
- Thyagarajan A, et al: Evidence of pathwayspecific basophil anergy induced by peanut oral immunotherapy in peanut-allergic children. Clin Exp Allergy 2012; 42: 1197–1205.
- Vickery BP, et al: Peanut oral immunotherapy modifies IgE and IgG4 responses to major peanut allergens. J Allergy Clin Immunol 2013; 131: 128–134.e1–e3.
- Gorelik M, et al: Suppression of the immunologic response to peanut during immunotherapy is often transient. J Allergy Clin Immunol 2015; 135: 1283–1292.
- Jones JC, et al: Incidence and risk factors associated with meniscal injuries among active- duty US military service members. J Athl Train 2012; 47: 67–73.
- Karlsson MR, Rugtveit J, Brandtzaeg P: Allergen- responsive CD4+CD25+ regulatory T cells in children who have outgrown cow’s milk allergy. J Exp Med 2004; 199: 1679–1688.
- Shreffler WG, et al: Association of allergenspecific regulatory T cells with the onset of clinical tolerance to milk protein. J Allergy Clin Immunol 2009; 123: 43–52.
- Smaldini PL, et al: Orally-induced intestinal CD4+ CD25+ FoxP3+ Treg controlled undesired responses towards oral antigens and effectively dampened food allergic reactions. PLoS One 2015; 10:e0141116.
- Begin P, Nadeau KC: Changes in peanut-specific T-cell clonotype with oral immunotherapy. J Allergy Clin Immunol 2015; 135: 1636–1638.
- Patil SU, et al: Peanut oral immunotherapy transiently expands circulating Ara h 2-specific B cells with a homologous repertoire in unrelated subjects. J Allergy Clin Immunol 2015; 136: 125–134.e12.
- Burton OT, et al: Oral immunotherapy induces IgG antibodies that act through FcγRIIb to suppress IgE-mediated hypersensitivity. J Allergy Clin Immunol 2014; 134: 1310–1317.e6.
- Frischmeyer-Guerrerio PA, et al: Modulation of dendritic cell innate and adaptive immune functions by oral and sublingual immunotherapy. Clin Immunol 2014; 155: 47– 59.
- Perezabad L, et al: Clinical efficacy and immunological changes subjacent to egg oral immunotherapy. Ann Allergy Asthma Immunol 2015; 114: 504–509.
- Blumchen K, et al: Oral peanut immunotherapy in children with peanut anaphylaxis. J Allergy Clin Immunol 2010; 126: 83–91.
- Varshney P, et al: A randomized controlled study of peanut oral immunotherapy: clinical desensitization and modulation of the allergic response. J Allergy Clin Immunol 2011; 127: 654–660.
- Anagnostou K, et al: Assessing the efficacy of oral immunotherapy for the desensitisation of peanut allergy in children (STOP II): a phase 2 randomised controlled trial. Lancet 2014; 383: 1297–1304.
- Narisety SD, et al: A randomized, doubleblind, placebo-controlled pilot study of sublingual versus oral immunotherapy for the treatment of peanut allergy. J Allergy Clin Immunol 2015; 135: 1275–1282.e1–e6.
- Tang ML, et al: Administration of a probiotic with peanut oral immunotherapy: a randomized trial. J Allergy Clin Immunol 2015; 135: 737–744 e8.
- Patriarca G, et al: Food allergy in children: results of a standardized protocol for oral desensitization. Hepatogastroenterology 1998; 45: 52–58.
- Patriarca G, et al: Oral desensitizing treatment in food allergy: clinical and immunological results. Aliment Pharmacol Ther 2003; 17: 459–465.
- Buchanan AD, et al: Egg oral immunotherapy in nonanaphylactic children with egg allergy. J Allergy Clin Immunol 2007; 119: 199– 205.
- Vickery BP, et al: Individualized IgE-based dosing of egg oral immunotherapy and the development of tolerance. Ann Allergy Asthma Immunol 2010; 105: 444–450.
- Staden U, et al: Specific oral tolerance induction in food allergy in children: efficacy and clinical patterns of reaction. Allergy 2007; 62: 1261–1269.
- Morisset M, et al: Oral desensitization in children with milk and egg allergies obtains recovery in a significant proportion of cases. A randomized study in 60 children with cow’s milk allergy and 90 children with egg allergy. Eur Ann Allergy Clin Immunol (Paris) 2007; 39: 12–19.
- Caminiti L, et al: Oral immunotherapy for egg allergy: a double-blind placebo-controlled study, with postdesensitization follow- up. J Allergy Clin Immunol Pract 2015; 3: 532–539.
- Escudero C, et al: Early SUSU after shortcourse egg oral immunotherapy: a randomized controlled study in egg-allergic children. Clin Exp Allergy 2015; 45: 1833–1843.
- Meglio P, et al: A protocol for oral desensitization in children with IgE-mediated cow’s milk allergy. Allergy 2004; 59: 980–987.
- Meglio P, et al: Oral desensitization in children with immunoglobulin E-mediated cow’s milk allergy – follow-up at 4 years and 8 months. Pediatr Allergy Immunol 2008; 19: 412–419.
- Skripak JM, et al: A randomized, doubleblind, placebo-controlled study of milk oral immunotherapy for cow’s milk allergy. J Allergy Clin Immunol 2008; 122: 1154–1160.
- Narisety SD, et al: Open-label maintenance after milk oral immunotherapy for IgE-mediated cow’s milk allergy. J Allergy Clin Immunol 2009; 124: 610–612.
- Longo G, et al: Specific oral tolerance induction in children with very severe cow’s milkinduced reactions. J Allergy Clin Immunol 2008; 121: 343–347.
- Martorell A, et al: Oral desensitization as a useful treatment in 2-year-old children with cow’s milk allergy. Clin Exp Allergy 2011; 41: 1297–1304.
- Salmivesi S, et al: Milk oral immunotherapy is effective in school-aged children. Acta Paediatr 2013; 102: 172–176.
- Pajno GB, et al: Comparison between two maintenance feeding regimens after successful cow’s milk oral desensitization. Pediatr Allergy Immunol 2013; 24: 376–381.
- Keet CA, et al: The safety and efficacy of sublingual and oral immunotherapy for milk allergy. J Allergy Clin Immunol 2012; 129: 448– 455, 455.e1–e5.
- Rodriguez del Rio P, et al: Oral immunotherapy in children with IgE-mediated wheat allergy: outcome and molecular changes. J Investig Allergol Clin Immunol 2014; 24: 240– 248.
- Sato S, et al: Wheat oral immunotherapy for wheat-induced anaphylaxis. J Allergy Clin Immunol 2015; 136: 1131–1133.e7.
- Bloom KA, et al: Effect of heat treatment on milk and egg proteins allergenicity. Pediatr Allergy Immunol 2014; 25: 740–746.
- Chatchatee P, et al: Identification of IgE- and IgG-binding epitopes on alpha(s1)-casein: differences in patients with persistent and transient cow’s milk allergy. J Allergy Clin Immunol 2001; 107: 379–383.
- Cooke SK, Sampson HA: Allergenic properties of ovomucoid in man. J Immunol 1997; 159: 2026–2032.
- Martos G, et al: Mechanisms underlying differential food allergy response to heated egg. J Allergy Clin Immunol 2011; 127: 990–997.
- Kim JS, et al: Dietary baked milk accelerates the resolution of cow’s milk allergy in children. J Allergy Clin Immunol 2011; 128: 125– 131.
- Leonard SA, et al: Dietary baked egg accelerates resolution of egg allergy in children. J Allergy Clin Immunol 2012; 130: 473–480.e1.
- Goldberg MR, et al: Efficacy of baked milk oral immunotherapy in baked milk-reactive allergic patients. J Allergy Clin Immunol 2015; 136: 1601–1606.
- Leonard SA, Nowak-Wegrzyn A: Food protein- induced enterocolitis syndrome: an update on natural history and review of management. Ann Allergy Asthma Immunol 2011; 107: 95–101.
- Nadeau KC, et al: Rapid oral desensitization in combination with omalizumab therapy in patients with cow’s milk allergy. J Allergy Clin Immunol 2011; 127: 1622–1624.
- Schneider LC, et al: A pilot study of omalizumab to facilitate rapid oral desensitization in high-risk peanut-allergic patients. J Allergy Clin Immunol 2013; 132: 1368–1374.
- Wood RA, et al: A randomized, doubleblind, placebo-controlled study of omalizumab combined with oral immunotherapy for the treatment of cow’s milk allergy. J Allergy Clin Immunol 2016;137:1103–1110.e11.
- Begin P, et al: Safety and feasibility of oral immunotherapy to multiple allergens for food allergy. Allergy Asthma Clin Immunol 2014; 10: 1.
- Begin P, et al: Phase 1 results of safety and tolerability in a rush oral immunotherapy protocol to multiple foods using omalizumab. Allergy Asthma Clin Immunol 2014; 10: 7.
- Savilahti EM, et al: Use of IgE and IgG4 epitope binding to predict the outcome of oral immunotherapy in cow’s milk allergy. Pediatr Allergy Immunol 2014; 25: 227–235.
- Hofmann AM, et al: Safety of a peanut oral immunotherapy protocol in children with peanut allergy. J Allergy Clin Immunol 2009; 124: 286–291, 291.
- Varshney P, et al: Adverse reactions during peanut oral immunotherapy home dosing. J Allergy Clin Immunol 2009; 124: 1351–1352.
- Lucendo AJ, Arias A, Tenias JM: Relation between eosinophilic esophagitis and oral immunotherapy for food allergy: a systematic review with meta-analysis. Ann Allergy Asthma Immunol 2014; 113: 624–629.
- Keet CA, et al: Long-term follow-up of oral immunotherapy for cow’s milk allergy. J Allergy Clin Immunol 2013; 132: 737–739 e6.
- Elizur A, et al: Clinical and laboratory 2-year outcome of oral immunotherapy in patients with cow’s milk allergy. Allergy 2016; 71: 275–278.
- Paassilta M, et al: Children who were treated with oral immunotherapy for cows’ milk allergy showed long-term desensitisation seven y
- Otani IM, et al: Multiple-allergen oral immunotherapy improves quality of life in caregivers of food-allergic pediatric subjects. Allergy Asthma Clin Immunol 2014; 10: 25.
- Epstein Rigbi N, et al: Patient quality of life following induction of oral immunotherapy for food allergy. Pediatr Allergy Immunol 2015;27:263–268.
- Greenhawt MJ, Vickery BP: Allergist-reported trends in the practice of food allergen oral immunotherapy. J Allergy Clin Immunol Pract 2015; 3: 33–38.
- Wasserman RL, et al: Oral immunotherapy for peanut allergy: multipractice experience with epinephrine-treated reactions. J Allergy Clin Immunol Pract 2014; 2: 91–96.
- Sampson HA: Peanut oral immunotherapy: is it ready for clinical practice? J Allergy Clin Immunol Pract 2013; 1: 15–21.
- Nurmatov U, et al: Effectiveness and safety of orally administered immunotherapy for food allergies: a systematic review and metaanalysis. Br J Nutr 2014; 111: 12–22.
- Sampson HA, et al: Food allergy: a practice parameter update-2014. J Allergy Clin Immunol 2014; 134: 1016–1025.e43.