Nurturing preterm infants-
Transition to effective feeding strategies
Univ.Prof. Nadja Haiden; MD,MSc, MBA
Advancements in neonatal intensive care have markedly improved the survival of extremely low birth weight (ELBW) infants and reduced the incidence of severe postnatal growth restriction. However, despite these achievements, many preterm infants are discharged from hospital with suboptimal nutritional status and growth trajectories that differ significantly from those of term infants. The post-discharge period therefore represents a critical phase in which nutrition must transition from medically controlled regimens to family-managed feeding, while continuing to meet the infant’s unique physiological and developmental needs. Optimizing growth during this period is fundamental for favorable neurodevelopmental outcomes and long-term metabolic health, yet remains challenging because of feeding difficulties, inconsistent nutrient intake, and the lack of standardized follow-up protocols.
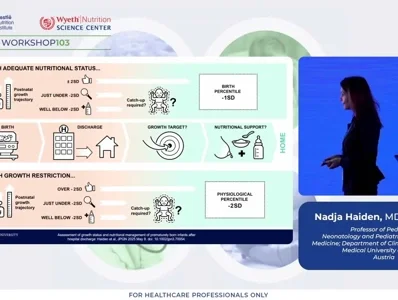
Effective post-discharge management requires meticulous anthropometric monitoring and individualized nutritional adaptation. Routine assessment of weight, length, and head circumference (HC) is essential to detect early deviations from expected growth patterns. Longitudinal evaluation of z-scores and growth velocity provides greater diagnostic precision than isolated measurements. Weight-for-length z-scores should be calculated at term-equivalent age to identify disproportionate growth patterns. Infants demonstrating post-discharge growth decline, defined as a fall in weight or length exceeding −2 standard deviations, warrant tailored nutritional interventions to promote catch-up growth and prevent long-term deficits.
Breast milk remains the optimal source of nutrition for preterm infants owing to its immunological and developmental benefits. However, because its nutrient density may be insufficient to sustain rapid postnatal growth in very preterm or growth-restricted infants, fortification should continue beyond discharge whenever feasible. When breastfeeding is not possible, nutrient-enriched post-discharge formulas with adequate protein-to-energy ratios and balanced mineral and trace element contents should be used to support appropriate growth and body composition.
The introduction of complementary foods constitutes another critical component of post-discharge nutrition. Current evidence suggests that the timing of solid food introduction between the 10th week and 6 months of corrected age does not significantly influence growth outcomes in the first year of life. Nevertheless, the process should be individualized and guided primarily by developmental readiness, including postural control and oral-motor coordination. Data on baby-led weaning in preterm infants are insufficient, and conventional spoon-fed approaches remain recommended until safety and efficacy are further established. Complementary foods should be nutrient-dense, emphasizing iron-rich options to mitigate the high risk of iron deficiency observed in this population.
Micronutrient supplementation remains fundamental to post-discharge care. Iron should be provided to all preterm infants according to birth weight and growth trajectory, with ferritin-guided adjustments during the first 6–12 months of corrected age. Vitamin D supplementation at 400 IU/day is recommended for all preterm infants, in accordance with current guidelines for term infants, with upper limits of 800–1000 IU/day for ELBW infants or those at risk of metabolic bone disease. Regular biochemical monitoring is advised to maintain serum 25-hydroxyvitamin D concentrations above 20 ng/mL (50 nmol/L).
In conclusion, individualized nutritional strategies and systematic follow-up are essential for optimizing post-discharge growth in preterm infants. Comprehensive anthropometric surveillance—including weight, length, HC, z-scores, and growth velocity—combined with appropriate use of preterm and WHO growth charts facilitates early recognition of growth faltering. Infants at high nutritional risk should be managed within specialized pediatric nutrition programs or by clinicians experienced in preterm follow-up. Ensuring adequate intake of protein, iron, and vitamin D, together with the timely introduction of complementary foods, underpins healthy growth and neurodevelopment in this vulnerable population. Further research should address existing knowledge gaps, particularly regarding the safety and long-term outcomes of alternative weaning methods such as baby-led weaning in preterm infants.
References:
Assessment of growth status and nutritional management of prematurely born infants after hospital discharge: A position paper of the ESPGHAN Nutrition Committee.
Haiden N, Luque V, Domellöf M, Hill S, Kivelä L, de Koning B, Kӧglmeier J, Moltu SJ, Norsa L, De Pipaon MS, Savino F, Verduci E, Bronsky J.J Pediatr Gastroenterol Nutr. 2025 Aug;81(2):421-441. doi: 10.1002/jpn3.70054. Epub 2025 May 8
Fenton Third-Generation Growth Charts of Preterm Infants Without Abnormal Fetal Growth: A Systematic Review and Meta-Analysis.
Fenton TR, Elmrayed S, Alshaikh BN.Paediatr Perinat Epidemiol. 2025 Aug;39(6):543-555. doi: 10.1111/ppe.70035. Epub 2025 Jun 19
Complementary Feeding: A Position Paper by the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) Committee on Nutrition.
Fewtrell M, Bronsky J, Campoy C, Domellöf M, Embleton N, Fidler Mis N, Hojsak I, Hulst JM, Indrio F, Lapillonne A, Molgaard C.J Pediatr Gastroenterol Nutr. 2017 Jan;64(1):119-132. doi: 10.1097/MPG.0000000000001454
Enteral Nutrition in Preterm Infants (2022): A Position Paper From the ESPGHAN Committee on Nutrition and Invited Experts.
Embleton ND, Jennifer Moltu S, Lapillonne A, van den Akker CHP, Carnielli V, Fusch C, Gerasimidis K, van Goudoever JB, Haiden N, Iacobelli S, Johnson MJ, Meyer S, Mihatsch W, de Pipaon MS, Rigo J, Zachariassen G, Bronsky J, Indrio F, Köglmeier J, de Koning B, Norsa L, Verduci E, Domellöf M.J Pediatr Gastroenterol Nutr. 2023 Feb 1;76(2):248-268. doi: 10.1097/MPG.0000000000003642. Epub 2022 Oct 28.
Iron requirements of infants and toddlers.
Domellöf M, Braegger C, Campoy C, Colomb V, Decsi T, Fewtrell M, Hojsak I, Mihatsch W, Molgaard C, Shamir R, Turck D, van Goudoever J; ESPGHAN Committee on Nutrition.J Pediatr Gastroenterol Nutr. 2014 Jan;58(1):119-29. doi: 10.1097/MPG.0000000000000206.PMID: 24135983