Mindful Microbes: The Interplay Between Environment, Gut Microbiome, Brain, and Behavior
Rebecca Knickmeyer
Infancy and early childhood represent the most dynamic phase of postnatal brain development—a period marked by rapid myelination, exuberant synaptogenesis, programmed cell death, and extensive synaptic and axonal pruning. These processes drive dramatic changes in neuroimaging phenotypes as well as in cognitive, emotional, and social abilities (Gilmore, Knickmeyer, & Gao, 2018). At the same time, the gut microbiome—a complex community of microorganisms capable of influencing the brain through the production of short-chain fatty acids, neurotransmitters, and immune-modulating metabolites—undergoes rapid assembly and maturation (Stewart et al., 2018). Although the microbiome’s ability to affect brain function and behavior is now well established (Sharon, Sampson, Geschwind, & Mazmanian, 2016), relatively little is known about how this gut-brain communication system develops during infancy and toddlerhood, despite strong evidence that early alterations in brain development can have lifelong consequences for learning and mental health.
A growing body of research links early gut colonization patterns to behavioral and neurobiological outcomes, including cognition, communication, social skills, temperament, stress responses, regional brain volumes, and functional connectivity. Dr. Knickmeyer will present findings from several key studies conducted by her team in this area.
One study examined whether gut microbiome composition at 12 months predicts cognitive and brain outcomes at 12 and 24 months. Among 89 typically developing infants, three distinct microbiome clusters were identified. Higher Mullen cognitive scores at age 2 were associated with lower alpha diversity at age 1 and with membership in a cluster characterized by high levels of Bacteroides. Infants in this cluster were also more likely to have been breastfed and delivered vaginally (Carlson et al., 2018). Alpha diversity was further associated with differences in brain connectivity measured by resting-state fMRI (Gao et al., 2019).
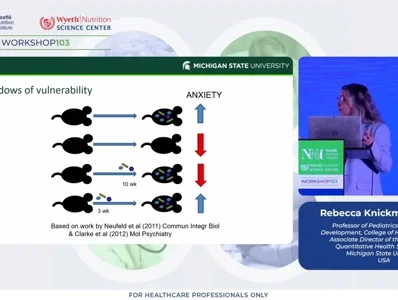
A subsequent pilot study investigated links between gut microbiome features at 1 month and 1 year, fear reactivity, and brain structure. Greater microbial diversity at 1 month predicted stronger HPA axis activation and heightened fear responses, while higher Bacteroides and lower Veillonella, Dialister, Bifidobacterium, and Lactobacillus at 12 months were associated with lower fear and smaller amygdala volumes (Carlson et al., 2021; Rosin et al., 2021). However, while significant associations were observed in a recently conducted replication attempt, the pattern of results differed. Variations in microbiome composition and fear response patterns between cohorts limited direct replication, underscoring the need for larger, multi-cohort studies.
Because most human infant studies are observational, causal relationships between the gut microbiome and neurodevelopmental outcomes remain to be established. In addition, MRI resolution limits make it difficult to pinpoint the specific neural processes involved. To address these gaps, Dr. Knickmeyer’s team recently transplanted microbiomes from human infants into germ-free pregnant mice to examine effects on offspring brain development and behavior (Dubey et al., 2023). Two communities commonly found in infants were tested—one dominated by Bifidobacterium and another by Bacteroides. Microbiome group influenced anxiety- and depression-related behaviors, as well as exploratory activity, and affected white matter integrity, dendritic complexity, and inter-regional brain communication.
In summary, early microbiome composition may represent a modifiable risk or protective factor for learning and long-term mental health. Immature gut communities characterized by lower diversity and greater Bifidobacterium abundance may promote cognitive development by supporting brain connectivity and conduction efficiency, whereas more mature, Bacteroides-rich communities may help infants regulate fear responses. Additional research is required to determine how personalized feeding guidelines, probiotics and prebiotics, and other intervention strategies targeting the microbiome can support both cognitive development and appropriate levels of fear reactivity.
References
Carlson, A. L., Xia, K., Azcarate-Peril, M. A., Goldman, B. D., Ahn, M., Styner, M. A., . . . Knickmeyer, R. C. (2018). Infant Gut Microbiome Associated With Cognitive Development. Biological Psychiatry, 83(2), 148-159. doi:10.1016/j.biopsych.2017.06.021
Carlson, A. L., Xia, K., Azcarate-Peril, M. A., Rosin, S. P., Fine, J. P., Mu, W., . . . Knickmeyer, R. C. (2021). Infant gut microbiome composition is associated with non-social fear behavior in a pilot study. Nat Commun, 12(1), 3294. doi:10.1038/s41467-021-23281-y
Dubey, H., Roychoudhury, R., Alex, A., Best, C., Liu, S., White, A., . . . Knickmeyer, R. (2023). Effect of Human Infant Gut Microbiota on Mouse Behavior, Dendritic Complexity, and Myelination. bioRxiv. doi:10.1101/2023.10.24.563309
Gao, W., Salzwedel, A. P., Carlson, A. L., Xia, K., Azcarate-Peril, M. A., Styner, M. A., . . . Knickmeyer, R. C. (2019). Gut microbiome and brain functional connectivity in infants-a preliminary study focusing on the amygdala. Psychopharmacology, 236(5), 1641-1651. doi:10.1007/s00213-018-5161-8
Gilmore, J. H., Knickmeyer, R. C., & Gao, W. (2018). Imaging structural and functional brain development in early childhood. Nature Reviews Neuroscience, 19(3), 123-137. doi:10.1038/nrn.2018.1
Rosin, S., Xia, K., Azcarate-Peril, M. A., Carlson, A. L., Propper, C. B., Thompson, A. L., . . . Knickmeyer, R. C. (2021). A preliminary study of gut microbiome variation and HPA axis reactivity in healthy infants. Psychoneuroendocrinology, 124, 105046. doi:10.1016/j.psyneuen.2020.105046
Sharon, G., Sampson, T. R., Geschwind, D. H., & Mazmanian, S. K. (2016). The Central Nervous System and the Gut Microbiome. Cell, 167(4), 915-932. doi:10.1016/j.cell.2016.10.027
Stewart, C. J., Ajami, N. J., O'Brien, J. L., Hutchinson, D. S., Smith, D. P., Wong, M. C., . . . Petrosino, J. F. (2018). Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature, 562(7728), 583-588. doi:10.1038/s41586-018-0617-x