NNI News - Update: Allergies, Proteins, Malnutrition
Allergies, obesity and healthy nutrition remain essential topics of everyday paediatric practice. And it is precisely in these areas that the recent research has yielded significant new findings. The key points to be mentioned in this publication are cow‘s milk allergy and FPIES, protein reduction in infant formula, human milk oligosaccharides as well as malnutrition. One focus is on the first months of life. It is well known that nutrition in the first 1,000 days, but also during childhood and adolescence, has long-term consequences for a healthy outcome in later life.
Early introduction of allergenic foods
Based on the LEAP study (Learning Early About Peanut), the early introduction of the peanut even in high-risk patients is now widely accepted. But most food allergies are caused by other triggers. This overview discusses the different approaches and studies.
Skin barrier: The role of an impaired skin barrier for food sensitisation and allergies has led to a number of dermatologically oriented studies. Despite the positive outlook of the BEEP study (Barrier Enhancement for Eczema Prevention) and a Japanese study, the large BEEP study and the PreVALL study (EuroPreVALL outpatient clinic study on food allergy) have not shown a significant effect of dermatological prevention on the risk of food allergies.
Environmental influences: The influence of environmental allergens on food allergies is supported by several peanut-related studies. However, it is far more difficult for a family to avoid allergens such as cow’s milk or egg than it is to avoid peanuts.
Other allergies: Of the studies on the early introduction of allergenic foods other than peanuts, 5 treat only the egg, 1 only milk, and EAT (Enquiring About Tolerance) (peanut, egg, milk, sesame, fish and wheat) and PreventADALL (Preventing Atopic Dermatitis and ALLergies) (peanut, milk, wheat, egg) treat several.
Egg: In their meta-analysis of the early introduction studies, Boyle et al (Ierodiakonou D et al., 2016) found in 5 studies (N = 1,915) that the early introduction of eggs at the age of 4 to 6 months was associated with a reduced egg allergy. Since the prevalence of a peanut allergy in the general population is significantly lower than that of an egg allergy, a programme that recommends early introduction of eggs provides much greater public health benefits.
Maternal diet: A systematic review of 42 studies found no consistent links between the nutrition of the mother and atopic diseases in their children. The now ongoing PrEggNut-RCT investigates whether a regularly high egg and peanut butter diet during pregnancy can prevent the development of a food allergy at the age of 12 months.
Domestic pets: Two studies have now observed a reduction in food allergies in the first year of life when a dog is kept in the household. In the HealthNuts study, children with a domestic dog were less likely to have egg allergies when they turn one year old.
Many countries and organisations have adopted a recommendation for the early introduction of a wide range of allergenic foods in early childhood, but other institutions still lack sufficient evidence of early introduction.
However, the question remains whether early consumption is food-dependent and whether the required starting age for this varies. Furthermore, it is not known what the consequences are if consumption is started but then only continues sporadically or stops completely. The recommended dose in each case also remains uncertain.
In clinical practice, the change in the assessment of the early introduction of allergens is now tangible. Families are also increasingly following a strategy of early exposure rather than avoidance. According to the authors, it will be interesting to see whether this leads to a general cultural change with regard to allergenic foods, which in itself could be a relief.
Perkin MR et al., J Allergy Clin Immunol Pract. 2020
Allergens Intro-Obstacles
In the EAT (”Enquiring About Tolerance“) study on the early introduction of six allergens, the rejection rate was surprisingly high, e.g. 42 % in the youngest group. The participants were therefore asked to give the reasons for this in an open online text questionnaire. Three groups of topics proved to be particularly challenging.
The answers for the analysis represent the early (4, 5 and 6 months), middle (8 and 12 months) and late (24 and 36 months) periods. The responses were coded and then grouped to represent key botanicals.
The three major challenges or obstacles during the early introduction of allergens proved to be
- Some children refused to eat the allergenic food,
- Parents feared an allergenic effect
- Practical difficulties or inconvenience during preparation
The authors conclude that an understanding of these complications requires corresponding communication and advice from doctors and nursing staff.
Voorheis P et al., J Allergy Clin Immunol, 2019
Is there a Connection between fruit and pollen allergy?
Fruits are considered to be common allergens. However, there is relatively little data on allergens that cause fruit and vegetable allergies and pollen food allergy syndrome (PFAS) in children. A Japanese multicentre observational study investigated the possible association between fruit/vegetable allergies and PFAS.
Among the participants were children aged < 15 years who developed allergic symptoms after eating fruit and vegetables and were subsequently treated in the paediatric department of six hospitals in Osaka Prefecture between August 2016 and July 2017.
A total of 97 children (mean age 9 years; 56 males) were included in the study. Apple was the most common allergen, followed by peach, kiwi, cantaloupe and watermelon.
74 participants (76%) had allergic symptoms due to PFAS; in addition, pathogenesis-related protein-10* (PR-10) was the most common allergen group. In the group without PR-10 or profilin sensitization, kiwi and banana were the most common allergens. The age of onset was lower than in the PFAS group. The specific antibody titre was significantly associated with birch for Bet v1 and latex for Bet v2 (r = 0.99 and r = 0.89).
The recommendation is to initially consider PFAS for patients with fruit and vegetable allergies, especially for children over 4 years of age.
Takemura Y et al., Asia Pac Allergy. 2020
* PR-10 proteins indicate IgE antibody cross-reactivity between homologous allergens in pollen and various vegetables, nuts and fruits.
Serious differences in extensively hydrolysed food for the treatment of cow’s milk allergy (CMA)
Not all extensively hydrolysed infant foods (eHF) are equally effective! This is the result of an analysis of different samples of commercial eHF, for the therapy of (CMA).
For infants with a CMA, eHF is the recommended first choice. It is assumed that the safety and suitability of eHFs has been thoroughly tested. However, documented allergic responses to eHFs have led to the hypothesis that some products may not be sufficiently hydrolysed and/or may be contaminated with cow‘s milk protein.
76 samples of commercially available whey and casein-based eHFs (eHF-W and eHF-C) from nine different manufacturers from different countries were tested. The following parameters were analysed:
- Molecular weight distribution of peptides
- Immunogenic peptides or proteins (IPP) derived from beta-lactoglobulin (BLG) or casein
- The in vitro allergenicity induced by BLG
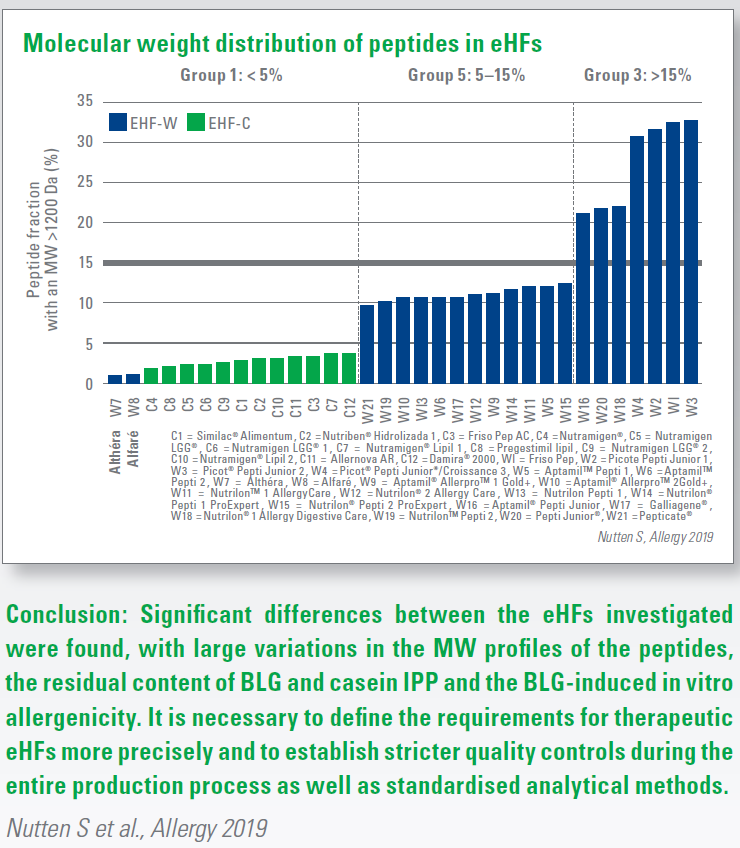
Results:
- The eHFs were divided into three groups according to their content of immunogenic peptides > 1,200 Dalton (DA): <5%, 5-15% and >15% (Fig. 1). 1,200 Da serves as a limit for the size of the peptides capable of binding an IgE molecule, which triggers the release of inflammatory mediators and cytokines.
- For three eHFs there was a significant difference in the MW profile between or within products of the same brand.
- In 22 of the eHFs a residual content of BLG-containing immunogenic peptides or proteins was found. 1 eHF-C and 2 eHF-W products tested positive for casein-derived immunogenic proteins and peptides.
- In 9 eHFs (2 eHF-C / 7 eHF-W) the content of peptides >1,200 Da could be associated with both the residual content of BLG-immunogenic peptides or proteins and the BLG-induced in vitro allergenicity.
Growth with a cow’s milk allergy
Children with a cow‘s milk allergy (CMA) are smaller and weigh less than those who suffer from peanut or walnut allergies. This fact remains until early adolescence. This finding is the result of a recent longterm study.
The data of paediatric patients with a persistent IgE-mediated allergy to cow‘s milk, peanuts or tree nuts were examined. The diagnosis was based on clinical symptoms, food specific IgE levels, prick tests and provocation tests. To be admitted, the children had to have at least one clinical examination during three defined time frames between 2 and 12 years, during which height and weight were also recorded
Between November 1994 and March 2015, 191 children were recorded,111 with CMA, 80 with nut allergies (61% and 51.3% male). The difference in size was more pronounced at the age of 5–8 and 9–12 years. For 53 children, whose data was recorded even >13 years, the difference was even more striking.
One explanation could be that children affected by CMA often have other allergies and therefore tend to avoid foods other than milk. Although the handling of CMA improves, it usually disappears during childhood. However, in children with persistent forms, greater attention should be paid to age-appropriate growth. Further studies are needed to find out which other factors play a role and to what extent the differences in height and weight remain in adulthood. Robbins et al., J Allergy 2020
HMO-enriched eHF for CMA is well tolerated
The aim of a study was to assess whether an extensively hydrolysed formula (eHF) enriched with 2 human milk oligosaccharides (HMO) is well tolerated by infants with cow‘s milk allergy and supports normal growth.
For the RCT study, infants, 0–6 months, with diagnosed CMA were enrolled at 44 sites. The test diet was a whey-based eHF with 2 Fucosyllactose (2‘FL) and Lacto-N-neotetraose (LNnT), protein content 2.20g/100 kcal. A currently marketed eHF (Althéra®, Nestlé Health Science) without HMO, protein content 2.47 g/100 kcal, served as a control food.
The infants received a milk-protein-free supplementary diet from the age of 4–6 months. Weight, height and head circumference were monitored monthly from admission (V0) to follow-up after 4. The primary study outcome was weight gain per day from V0 to V4. Secondary endpoints were Z-scores for weight for age, height for age and head circumference for age, as well as clinical tolerance, number of adverse events (AE) and drug use up to 12 months of age.
194 of the children examined were randomly switched to test (n=97) or control food (n=97). The mean age at V0 was 3.2 ± 1.7 months; 99 (51.0%) were male. At V4 the per-protocol cohort (PP) included 137 infants (73 test, 64 control). The daily weight gain of the test diet was no less from V0 to V4 than that of the control diet (19.38 g/d vs. 20.12 g/d; p= 0.0049). At no time were there significant group differences for any of the anthropometric parameters.
In the study group, the rate of lower respiratory tract infections in the test group between V0 and V6 was reduced by 38.1% (12/73 vs. 17/64); upper respiratory tract infections were reduced by 14.8% (35/73 vs. 36/64). Otitis media was reduced by 100% (0/73 vs. 7/64). The use of antibiotics was 20.3% lower in the study group (20/73 vs. 22/64) and of antipyretics 24.9% (30/73 vs. 35/64). However, the small sample size does not allow stable statistical conclusions about these secondary outcomes.
Conclusion: The infants fed with the HMOenriched test formula achieved normal growth. The PP analysis indicates a reduced risk of respiratory tract and ear infections as well as a reduced need for antibiotics and antipyretics. Vandenplas Y et al., PAAM 2019
Safety of AF food in practice
Special foods based on amino acids (AF) are used for children suffering from cow‘s milk allergy (CMA), multiple food allergies or malabsorption. They are recommended by the professional societies when extensively hydrolysed foods (eHF) are not effective.
The aim of a first post-market safety study with AF foods, was to examine the frequency and type of adverse events (AE) in children receiving AF foods. The second objective was to study the demographic and clinical characteristics of children with AE.
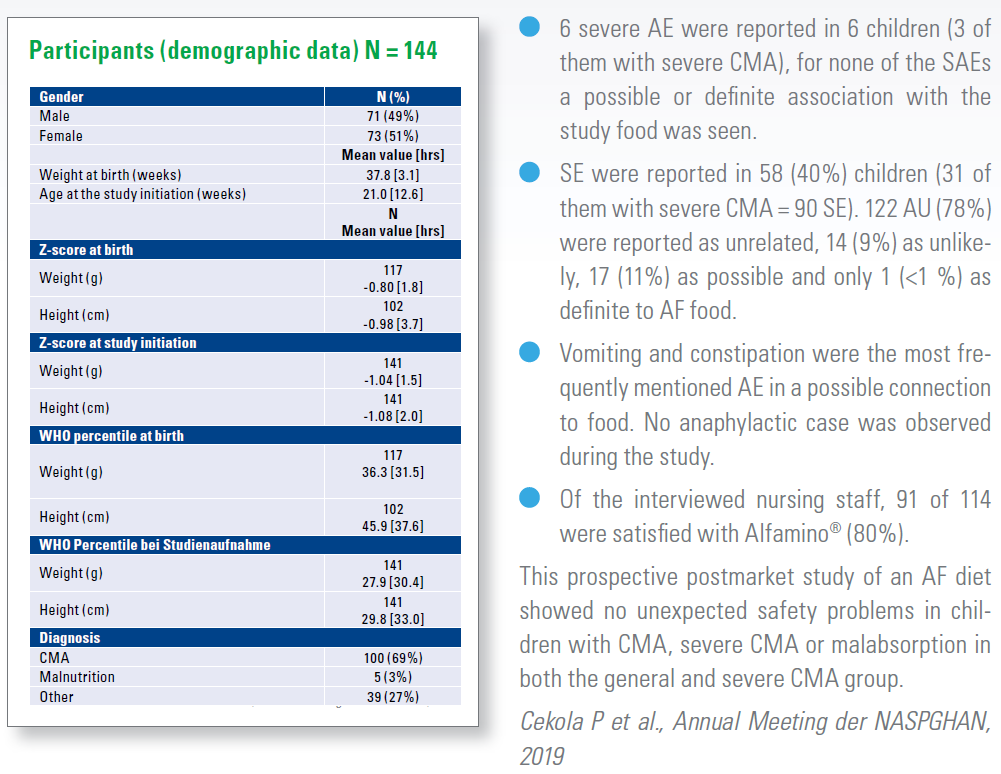
The study took place between February 2017 and May 2018 at 30 locations in the USA. Children at ≤ 12 months were enrolled and with a gestational age > 37 weeks. They received a uniform AF food (Alfamino® Infant) for 4 months. The data of 144 children were collected at the beginning, 88 could be followed over the 4 months. 100 (69%) had a diagnosed CMA, 84 of them were classified as severe CMA due to a medical diagnosis, anaphylaxis or the unsuccessful eHF use.
FPIES – an overview
With a cumulative incidence of 0.015 % to 0.7% in infants, food protein induced enterocolitis (FPIES) is not uncommon. An up-todate overview of epidemiology, pathophysiology, diagnosis and management.
FPIES (= food protein-induced enterocolitis syndrome) is a non-IgE-media- ted food allergy that manifests itself with repeated, erratic vomiting, which can be followed by diarrhoea and is accompanied by lethargy, hypotension, hypothermia, and metabolic disorders.

FPIES usually begins in childhood, usually with the beginning of supplementary feeding, although increasingly a beginning at a higher age is also recognised. Diagnosis is challenging because of a delay in the onset of symptoms after food intake of 1 to 4 hours, the absence of the typical allergic skin and respiratory symptoms and food triggers that are considered hypoallergenic. Because there are no biomarkers for FPIES, diagnosis is based on the recognition of symptoms. The pathophysiology remains unclear, although the activation of the innate immune response has been demonstrated.
Management is based on the avoidance of triggers, the treatment of unintentional exposures and periodic reassessment by oral provocation. There are no strategies to accelerate the development of tolerance in FPIES.
Nowak-Wegrzyn A et al., J Allergy Clin Immunol Pract 2020
Questions about the Nature of FPIES
Non-IgE-mediated food protein induced enterocolitis (FPIES) mainly affects young infants. The most common triggers are milk, soy and cereals. Studies evaluating the natural course of FPIES show heterogeneous tolerance levels.
Several factors influence these differences, including country of origin, food triggers, atopic status, reference populations and methodological differences in the studies.
FPIES to liquids (cow‘s milk and soya food) has a generally favourable course, most patients develop tolerance up to the age of 5 years, with solid food the tolerance development usually occurs later.
The subgroup of patients with atypical FPIES – i.e. with a positive specific IgE associated with the suspect foods - might have a more severe phenotype and slower tolerance development. Also, an increased risk could be due to an IgE-mediated phenotype.
Further multicentre and prospective studies are needed to better determine the natural course of FPIES in individual foods, to determine the optimal time of food introduction and to clarify the risk factors associated with persistent FPIES.
Graham F et al., in: Brown-Whitehorn T, Cianferoni A (eds), 2019
“Typical for FPIES is that the triggers are atypical.”
A discussion with senior physician Dr. Lars Lange, specialist for paediatric and adolescent medicine, paediatric pneumology and allergology at the GFO Bonn – Paediatrics St. Marien. He is currently working on a Germany-wide study on FPIES. Dr. Lange, recently there has been an increase in studies and articles on food protein induced enterocolitis. Is FPIES in vogue? Or is it simply diagnosed in greater numbers?
We believe that FPIES is diagnosed much more. It‘s more common to be diagnosed with a „Oh, he can‘t take it.“ This is for acute FPIES. Almost after every lecture and every event on the subject, someone with this experience comes forward.
In the case of chronic FPIES, everything was previously subsumed under the collective term „cow‘s milk protein intolerance“, which concerned cow‘s milk intolerance in infancy. Now there is a more precise differentiation into different clinical pictures. This is the fact that an example significant for the forecast. The trigger is always cow‘s milk, even in breastfed children the allergen in breast milk is cow‘s milk. The course of the form triggered by breast milk is relatively mild. In countries where soya is also fed early, it is also a trigger.
FPIES is recognized more often today.
Are there, similar to cow‘s milk allergy, no typical symptoms?
We are currently collecting cases of chronic and acute FPIES in a Germany-wide study. If you go through the doctor‘s letters, you often see clear FPIES cases, which unfortunately were not diagnosed in this way due to ignorance. If you stick to the diagnosis criteria, it is actually not that difficult. There are of course always borderline cases, and there is certainly a lot of leeway.
What signs suggest the possibility of a FPIES illness?
There are diagnostic criteria. In acute FPIES, repetitive vomiting typically occurs 1–4 hours after food intake. This period is individual for each patient, but always the same. In one patient it is 2 hours, in another 2.5 or even only 1.5 hours. If about the same amount is ingested, the vomiting per patient shows up at the same time. This vomiting is accompanied by severe symptoms, but without the typical IgE-mediated symptoms such as hives or the like. Further criteria are, for example, a severe course that requires a hospital stay, dehydration, changes in blood gas analysis due to the recurrent vomiting. According to this criteria catalogue FPIES can be diagnosed.
In chronic FPIES we have the clinical picture of a very, very sick infant under cow‘s milk or under breast milk nutrition. If the infants receive extensive hydrolysate nutrition they will be healthy. If they are exposed again, they become ill again – thus the diagnosis is confirmed.
Cow‘s milk has a good prognosis in children.
The beginning of the supplementary diet often seems to be a trigger?
In the major English EAT study on early induction of infectious diseases there were increased FPIES cases related to chicken eggs. It was suspected that early introduction leads to an increased FPIES propensity. No data are known on the early introduction of cow‘s milk. In the meantime, we have found that in Germany comparatively many children react to potatoes, but it is a rather nonallergenic food. There are also patients who react to meat and comparatively many to fish. The classics are oats and rice, which are rather rare allergens. But there are almost no FPIES cases on nuts in Europe. This also often makes the diagnosis difficult, but typical for FPIES is that they are atypical triggers.
Could a factor be an hereditary atopic strain, if allergies have already occurred in the immediate family?
FPIES is not a classical atopic disease, so it should not be a reinforcing factor. However, we do see a certain familial predisposition, e.g. in our study we have some sibling couples with FPIES.
There is also an atypical form of FPIES, which is probably IgE-mediated. A special clinical challenge?
There are different data for this. One study states that up to one third of the patients also developed IgE antibodies against their allergen, then only showed clinical FPIES symptoms, i.e. vomiting after 2–3 hours, but without wheals etc. According to this study, the patients would have a worse prognosis, we cannot confirm this. We had some children with FPIES and IgE whose prognosis was similar to the other cases. These children should be treated slightly differently and provoked differently later, but no fundamental change in therapy should be made.
What are the specific recommendations for therapy?
Strict avoidance of the corresponding allergen! Afterwards, depending on the food in question, regular exposures to clarify whether FPIES has disappeared. In the case of cow‘s milk, for example, provocation is recommended after about one year to clarify whether it has disappeared. With solid food you wait a little longer; you would perhaps wait 2–3 years.
In acute treatment there is a special case that one does not treat with adrenalin and antihistamines as in an allergic shock, but mainly with fluid substitution and the antiemetic ondansetron. This stops the vomiting within a very short time. However, it has to be used in a clinical setting, because it has to be given intravenously.
What can you say about the course of events? Is there any hope for the parents and the young patients?
Especially cow‘s milk has a very good prognosis in children, also with cereals FPIES disappears after some years, with fish it takes about 5–6 years for a chance of tolerance development. FPIES is also a phenomenon in adults and seafood is often not tolerated – but we do not have any figures on this..
With chronic FPIES the prognosis is good, after 1–2 years it usually disappears.
HMO in the nutrition of infants
A mother‘s breast milk is the natural and ideal food for babies. It provides the energy and nutrients that every infant needs in the first 4–6 months of life, in the right quality and quantity. Breastfeeding and the resulting intestinal microbiomes are related to the better health of the infant. HMOs play an important role here.
Many factors such as the length of lactation, environmental and genetic factors influence the amount of HMOs. HMOs can support the development of immune function and provide protection against infectious diseases, directly through the interaction of intestinal epithelial cells or indirectly through the modulation of intestinal microbiota, including the stimulation of bifidobacteria.
Infants who are breastfed for shorter periods or not breastfed suffer more frequently from infectious diseases such as gastroenteritis and acute middle ear infection, have more immune-mediated diseases, a lower intelligence quotient. And they are likely to have a higher risk of overweight and type 2 diabetes later in life.
Recently it has become possible to produce the first oligosaccharides that are structurally identical to those found in breast milk. It is important that HMOs are resistant to cold and heat and are not affected by pasteurisation and freeze-drying. The addition of an HMO, namely 2‘-fucosyllactose (2‘-FL), is a step forward in bringing artificial infant nutrition closer to the gold standard: breast milk.
No adverse effects have been reported for the addition of an HMO, namely 2‘-fucosyllactose (2‘-FL), and in vitro and animal studies have demonstrated the benefits of supplementing infant formula with 2‘-FL. Lacto-N-neotetraose (LnNT) is also added to the infant formula. A first clinical study with an infant formula (2‘-FL and LNnT) showed that infants fed with the 2‘-FL and LNnT formula were less likely to develop respiratory infections and needed antibiotics less often in the first year of life than those fed the same formula without HMO. From this it can be concluded that 2‘-FL and LNnT are a safe and beneficial supplement to infant formula.
This is especially true for special foods for infants with cow‘s milk allergy (CMA).
Vandenplas Y, DOHaD 2019
Microbiome and neurological development
Pregnancy and the first weeks of life are marked by significant changes in the composition of our body‘s microbiomes. These changes coincide with key periods of infant developmental plasticity.
This leads to a complex dialogue between the brain and the microbes that colonize the gastrointestinal tract. Studies in recent years have shown the existence of a bi-directional microbial gut-brain axis.
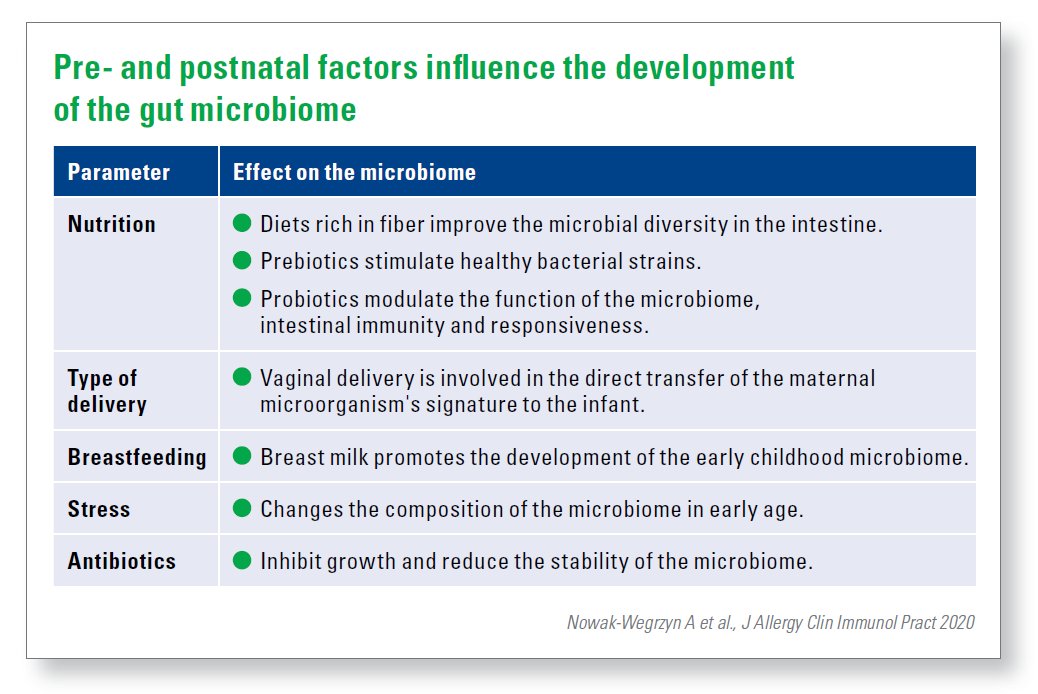
The composition of the intestinal microbiome of the pregnant woman is subject to a dynamic change to support the fetus. This composition is influenced by numerous external factors such as nutrition, medication, lifestyle, but also by infections, hospital stays and stress. Inadequate intake of micro and macronutrients, maternal overweight and high fat content are associated with poor development of the child‘s nervous system. New data indicate that maternal consumption of prebiotics, probiotics and psychotropic drugs affect the stability of the maternal and child‘s microbiome and thus the behavior of the offspring.
The type of delivery affects the infant‘s microbiome only temporarily. Over time, section children develop a microbial population similar to that of infants with vaginal deliveries.
Breastfeeding is obviously an influential parameter compared to bottle feeding. Breast-fed infants have a clear microbial „fingerprint“ in the intestine, they also show improved verbal and non-verbal cognitive skills in childhood compared to the toddlers who received infant formula.
Codagnone MG, Ann Nutr Metab 2019
2’FL promotes cognitive development
Maternal factors, such as breast milk, have been shown to influence the development of a baby. Animal studies have shown that the oligosaccharide 2‘FL contained in breast milk has a positive influence on the development of the nervous system. The first human study confirmed that this 2‘FL found in breast milk promotes cognitive development.
In a cohort study at the Saban Research Institute of Children‘s Hospital Los Angeles with 50 mothers and their babies, the researchers analysed the composition of breast milk and the frequency of feeding at the ages of 1 and 6 months. A special technology makes it possible to link differences in milk composition with specific infant outcomes such as cognitive development. Cognitive development was measured at the age of 24 months using the Bayley III scale. It was shown that the amount of 2‘FL in breast milk in the first month of feeding was associated with significantly higher values of cognitive development in infants at the age of 2 years. Since similar associations were not observed after 6 months, it can be assumed that early exposure to 2‘FL may be most important for the improvement of infant learning and memory, with benefits that may continue into adulthood.
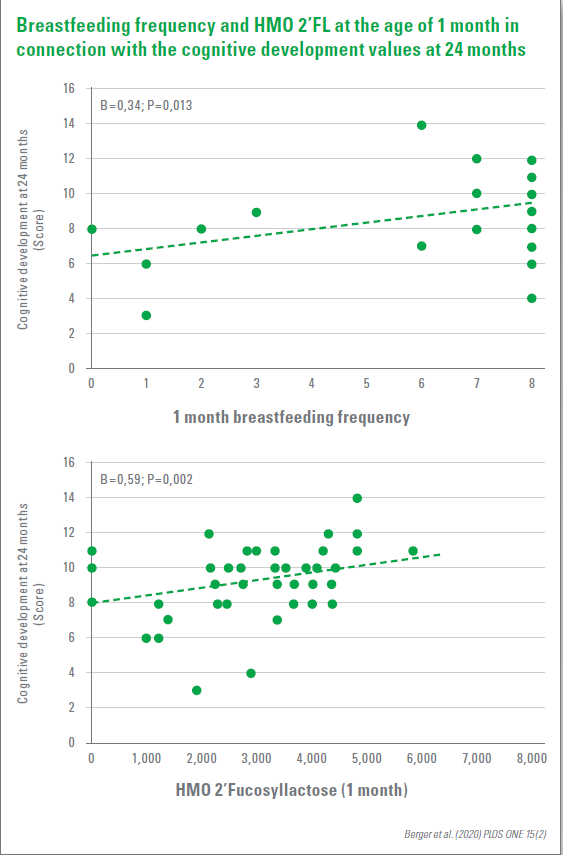
The observations showed that the improved neuronal development induced by breastfeeding is primarily due to mothers producing more 2‘FL for the baby‘s consumption. For women who are unable to breastfeed or can only breastfeed briefly, 2‘FL could be added to the baby‘s diet to support its cognitive development.
Berger PK et al., PLOS ONE, 2020
HMOs influence the risks of a premature birth
Every year around 15 million children are born too early. Premature birth is one of the main causes of new-born mortality. The causes of spontaneous preterm birth (PTB) are multifactorial and often unknown. The study tested the hypothesis that human milk oligosaccharides (HMOs) in blood and urine modulate the maternal urinary and vaginal microbiome and influence the risk for PTB.
The vaginal and urinary microbiome of a cross-sectional cohort of women with and without preterm labour was analysed and correlated with measurements of metabolites and HMO in urine and blood. Several microbial signatures associated with the short cervix, PTB and/or preterm contractions were identified, e.g. Lactobacillus jensenii, L. gasseri, Urea-plasma sp. and Gardnerella sp. In addition, associations between sialylated HMO, especially 3‘-sialylylactose, with PTB, short cervix and increased inflammation were shown. They confirmed an influence of HMO on the microbiome profile.
In the identification of serum and urine HMOs and several key chemical organisms associated with PTB, the results indicate two different processes which influence the risk for PTB.
- One process appears to be driven by sterile inflammation characterised by elevated serum concentrations of sialysed HMO.
- A second process could be microbially mediated, possibly driven by secretor-active HMOs in urine.
Pausan M-R et al., bioRxiv 2019
Protein reduced infant formula against obesity risk
The EU has now decided to lower the lower limit of the protein content in follow-on foods from 1.8 g to 1.6 g / 100 kcal. This means that children who are not breastfed can be offered an age-optimized step system that allows the use of follow-on nutrition with a reduced protein content as required.
The protein intake with infant formulas and follow-on foods therefore corresponds better to the protein intake during breastfeeding. In the course of early childhood development the nutritional requirements change, the protein requirement per kg body weight decreases continuously. The protein intake through breast milk is dynamic, it adapts to this changing need. It contains a relatively large amount of protein in the first few weeks, later its protein content is reduced (Fig. 1).
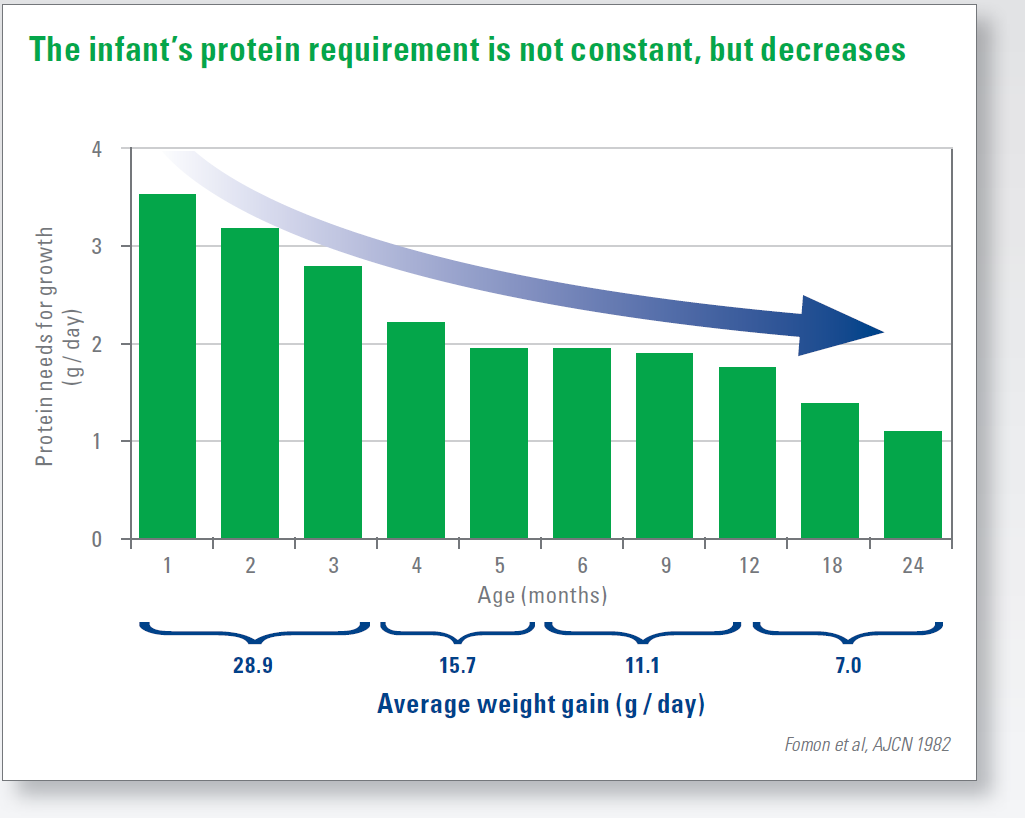
For a long time, inadequate protein intake and malnutrition were in the foreground. Especially in early childhood, an insufficient supply of protein leads to long-term health restrictions. For extremely malnourished children, a protein-rich diet is still essential.
However, excessive protein intake in infancy leads, among other things, to increased fat cell formation, accelerated weight gain and early programming of a long-term risk of obesity. This has been confirmed, for example, by the large European CHOP study (EU Childhood Obesity Project). A reduced protein intake in infants – by promoting breastfeeding as well as by lowering the protein content in baby foods – could thus contribute to a healthy weight development in the long term.
It is important to have a higher protein quality at the same time in order to ensure that, despite this reduction, all the ingredients that are important for the growth process, e.g. to provide the essential amino acids in an optimal composition.
https://www.nestlenutrition-institute.org/country/de/im-fokus/protein
New technology for clarification of macronutrients in breast milk
Breast milk is currently enriched for the nutrition of premature babies on the basis of an assumed macronutrient composition. However, this can lead to either over or under enrichment. Objective enrichment is limited by the lack of fast, accurate methods for measuring macronutrients. Can HMA technology help here?
The aim of a current study was to evaluate the accuracy and precision of the HMA (Mid-Infrared Human Milk Analyzer). The Röse-Gottlieb method, high-performance anion chromatography with pulsed amperometric detection (HPAECPAD), Kjeldahl and amino acid analysis (AA) were selected as reference methods for total fat, lactose and protein content.
No significant difference in lactose content between HMA and HPAEC-PAD was observed. In contrast, significant differences were observed in the fat and protein content between HMA and the reference methods. However, the difference in fat content was < 12% and was therefore within the variability specified by the supplier. The BCA protein test was selected for the protein determination. There were no significant differences in the total protein content measured with the BCA test, Kjeldahl and AA.
Conclusion: HMA was reliable for the quantification of the total fat and lactose content, but not for the total protein content. This was measured using the BCA test, which gave comparable results to the Kjeldahl and AA methods.
Giuffrida F et al., Journal of Perinatology 2018
“Greater protein content increases appetite”
A conversation with PD Dr Frank Jochum, CA of the Clinic for Child and Youth Medicine of the Protestant Forest Hospital Spandau and CA of the Clinic for Neonatology of the Martin-Luther-Hospital in Berlin-Wilmersdorf, President of the German Society for Nutritional Medicine e.V. (DGEM). A high protein intake in infancy seems to play a major role in the risk of developing obesity?
Yes, this is the case and the pathomechanism has been largely elucidated. Proteins consist largely of amino acids. The branched-chain amino acids valine, leucine and isoleucine are also part of the proteins. These have the property of stimulating insulin secretion. If a high protein intake with a high proportion of these amino acids takes place at an early age, the result is a slightly higher insulin release and thus a slightly lower blood sugar level. In simple terms, this also leads to a little more hunger. One eats slightly more and thus becomes overweight – a programming effect that lasts a lifetime.
This has an effect especially in infancy?
The first 1,000 days of life from conception play a special role. Even the diet of the pregnant woman has an effect on the foetus. After a birth, the metabolic processes are still adjusted and if malnutrition occurs during these phases, this can lead to long-term effects – as were just described.
With the new EU regulations, it should also be possible to lower the protein content of follow-on foods since February. What is behind it?
Until now, the postulate has been valid that infant food should not be allowed to cause a lack of essential amino acids. That is why the protein content was set rather high in the past. It had also not yet been understood that it is not good for infants to grow unphysiologically fast. For a long time it was believed that the faster growth and weight gain developed, the better the food. Today we know better.
This is linked to the requirement to use only „high-quality protein“. What does this mean?
A limiting factor used to be the amount of essential amino acids in infant formula, so that a certain safety margin was always added. Moreover, for a long time the individual amino acids could not be selectively increased, which is possible today. This is one of the reasons why a slightly higher protein content was always added.
Today it is possible to work with a significantly lower protein content. Essential amino acids can also be added specifically today. This also enables manufacturers to create other compositions.
The first 1,000 days have a very special role
The results of various studies, such as the large European CHOP study, have ultimately led to lower protein content of infant formulae?
The CHOP study is a good example of how relationships can be demonstrated. Even before this, the high protein intake at a certain age on the one hand and the later development of adiposity on the other hand had already been seen. But the pathomechanism was not known. This is unsatisfactory for us scientists, because we do not know whether cause and effect are really directly connected. It is the merit of the CHOP study not only to check associations, but also to define the pathomechanism as a hypothesis and then test it. In this way, we can also be sure that the new EU regulation is the right way forward.
The protein requirement of the growing infant decreases continuously. There is already a distinction between starter food and follow-up food. But wouldn‘t it make more sense to divide these into three or even more groups?
It would of course be interesting in times when breastfeeding is otherwise exclusively done to orientate oneself towards breast milk and to introduce an additional gradation for this purpose. This would be innovative and would better correspond to the development of breast milk.
Now, however, it is already frequently the case that the wrong food is fed or the wrong portions are portioned. Wouldn‘t this increase the complexity and error rate even more?
The portioning and dissolving of the powder is indeed a big problem, there are also studies on this. The manufacturers have come up with some ideas about dosage aids etc. but in my opinion this is not yet solved.
Is there not still a risk of malnutrition, for example in developing countries, but also in children with a low birth weight?
There are still areas of the world where not even the basic food supply is guaranteed, and unfortunately there are still people there who actually suffer from protein deficiency.
Children with a birth weight that is too low for the duration of pregnancy are anxious to reach their age-appropriate weight. This usually leads to a catch-up growth. This requires correspondingly more nutrients, i.e. also more protein. Especially when it comes to growth and development of tissue and organs, this is a very important point. This is where deficiencies can actually occur.
Premature babies are a special case. The protein requirement of premature babies is much higher. What are the recommendations here?
The foetus is fed intrauterine via the placenta, which means that nutrients are available as needed and without any limitations. Premature babies have a growth rate that is much higher than that of a mature baby, and thus have a higher nutrient and protein requirement. The more premature the baby is, the higher the growth rate and therefore the higher the need. In the past it was assumed that the faster premature babies grow and thus make up the deficit, the better it would be.
Today we know that this is associated with an adjustment of the metabolic pathways and the regulatory circuits, such as the insulin-glucose system.
On the other hand, if they grow more slowly, this permanently inhibits neurological development, which cannot be made up for later. It is therefore necessary to supply the amounts of nutrients and protein so that it corresponds as closely as possible to intrauterine growth.
In neonatalogy, we are now able to do this relatively well. But there‘s also air up there....
Lack and abundance – differentiated
Malnutrition in the first years of life has immediate health disadvantages and impairs health and performance in the long term. Children born too small and those who are malnourished at a young age a re more susceptible to disease and have a higher risk of dying prematurely.
Poor growth is associated with various physical and cognitive consequences and affects over 150 million children worldwide. More than 50 million children are atrophied, half of them live in South Asia. But 5.4 million of the 38.3 million overweight or obese children in the world also live there. There is growing evidence that being overweight at an early age is associated with the risk of noncommunicable, e.g. cardiovascular, diseases later in life.
Although the worldwide prevalence of growth retardation decreased from 32.6 % in 2000 to 22.2 % in 2017, this reduction varies considerably from region to region (Asia: 38.1–23.2 %; Africa: 38.3–30.3 %; Latin America: 15.9–9.6 %). In a few countries, such as Nepal (57.1-36.0%) and Lesotho (52.7–33.4%), dramatic progress has been made.
Similarly, the prevalence of childhood obesity varies widely by region, as does the prevalence of childhood obesity worldwide, which increased between 2000 and 2017. Here, the largest increases have been in Eastern Europe and Central Asia (8.2–14.8%). Only West and Central Africa recorded a decrease from 4.3% to 3.0%. If these trends continue, 70 million children will be overweight or obese by 2025. These global, national and regional statistics are crucial for tracking progress and informing policy and programme priorities.
However, the implementation of specific measures should be based on detailed data to better understand which population groups are most affected and the underlying causes. Analyses from India, India and Vietnam, for example, suggest that the determinants of growth inhibition vary across the geography of the countries. In India, for example, the prevalence of growth inhibition, broken down by district, is between 12.4% and 65.1%.
This variability should not be surprising, as all forms of early life malnutrition have a complex aetiology.
Taking India as an example, the variety of factors includes geography (higher in the north and in the middle), level factors (wealth, household size), maternal factors (care during pregnancy, BMI, age of marriage, education), and filial factors (adequacy of nutrition). These factors explain 71 % of the variance in the prevalence of malnutrition, but 29 % remain unexplained.
For decades, anaemia was considered the only indicator of malnutrition worldwide. But anaemia also has a complex aetiology and numerous nonnutritional causes. Unfortunately, such causes are rarely recorded on a larger scale. However, this would be necessary for targeted measures to achieve improvements for all forms of malnutrition.
Neufeld LM et al., 93rd Nestlé Nutrition Institute Workshop, Kalkutta, 2019
Malnutrition in the intensive care unit
Adequate food intake is essential during the stay in the paediatric intensive care unit. It was investigated whether the achievement of energy intake targets on day 4 after ingestion and the form of food intake were associated with an improved outcome.
Inadequate nutrition leads to energy and protein deficits, which result in insufficient weight and growth. In addition, malnutrition is associated with increased morbidity and mortality in seriously ill children. The prevalence of acute malnutrition, defined as weight-for-age < -2 SD, affects almost a quarter of all patients in the paediatric intensive care unit (PICU) and has hardly improved in recent decades.
Nutrition and outcome in 325 patients at the PICU, enteral and/or parenteral nutrition was included. The goal of energy intake the day after admission was 90–110% of the energy requirement at rest.
An observational study with prospectively obtained data reviewed acute malnutrition was defined as weight-for-age <-2 SD. The duration of stay, the days on the ventilator, the duration of antibiotic administration and the number of new infections were recorded.
Of the subjects (age 0.14 (0.0–18.0 years), 19% were acutely malnourished at admission.
In the median, 86% of the energy targets were administered enteral. With enteral energy delivery, 7% of patients were fed within the target frame, 50% below and 43% above the target frame. Acute malnutrition was observed very frequently at admission and discharge. In a subgroup (n=223) the acute malnutrition rate at discharge (26%) did not differ significantly from that at admission (22%).
However, there was no correlation between the amount of energy intake or the type of food intake and the clinical outcome.
de Betue CTI et al., Clinical Nutrition 2015
Iron supplementation in the first years of life
The first 1,000 days in a child‘s life are crucial for the developing brain. During this time, iron deficiency has far-reaching consequences, especially affecting cognitive development.
There is therefore great interest in preventing or treating anaemia in pregnancy and during the first 2 years of life. However, there is a lack of meaningful data from high-quality randomised studies investigating the effects of various iron interventions during pregnancy, infancy and early childhood on cognitive development. Despite decades of research, there is a lack of conclusive evidence for the optimal strategy for treating iron deficiency in infants and children. The varying results from different studies may be due to variations in dosage, duration and timing of iron treatment and basic characteristics of the study population. While there is clear evidence of the benefits of iron supplementation in cognitive performance in children who are anaemic, at school age. Studies in pre-school children have shown mixed results for visual, cognitive and psychomotor development. The evidence for basic iron supplementation in children under 2 years of age remains unclear. Some studies show small benefits, while others show no differences compared to placebo.
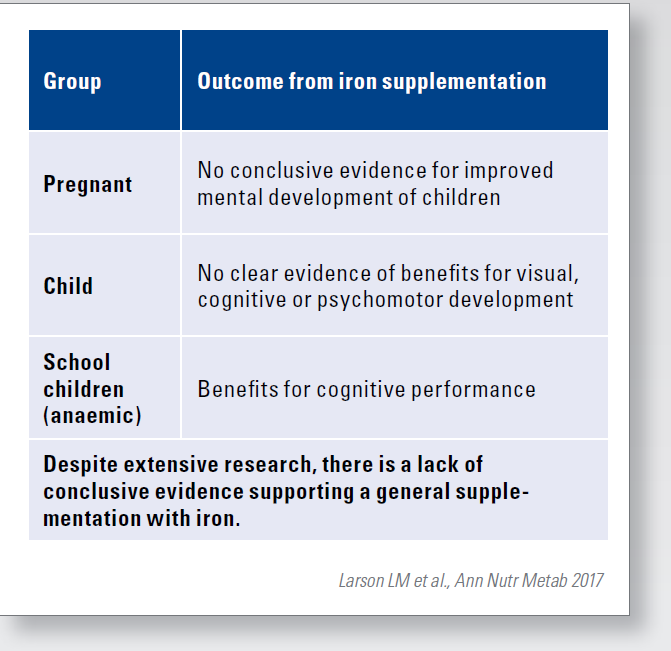
Caution should also be exercised when exceeding the recommended doses. This is because some studies report negative effects of high iron accumulation.
Larson LM et al., Ann Nutr Metab 2017
Iron deficiency has long-term effects
Iron deficiency is the most widespread deficiency of micronutrients worldwide. There is evidence that iron deficiency causes changes in the developing brain, which eventually lead to long-term effects on various cognitive functions.
A long-term Chilean research project investigated motor learning and its consolidation after sleep in adolescents who had persistent iron deficiency anaemia (IDA) in infancy.
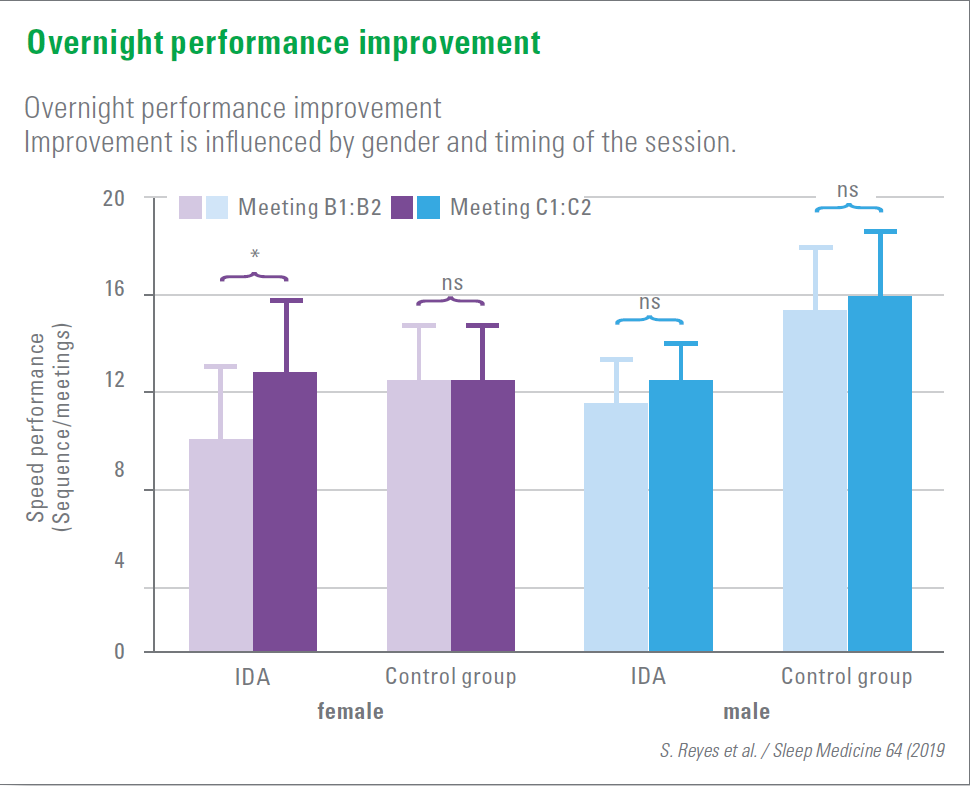
53 adolescents (15–16 years) who had had proven anaemia as infants and 40 control adolescents practiced sequential motor finger tapping before and after a night‘s sleep. Performance was measured at the end of learning, 30 minutes later (boost effect) and the next morning.
It was shown that adolescents with iron deficiency anaemia learned more slowly in infancy than those in the control group, followed by a proportionally similar increase in performance after 30 minutes. Overnight performance remained stable in the control group but improved in the IDA adolescents. This suggests a positive effect of a post-training sleep to consolidate the incompletely learned motor skills. The overnight improvement in performance was observed mainly in the female IDA adolescents, but not in the male ones. This indicates a gender effect.
These results indicate long-lasting motor learning deficits, which sleep after training could compensate for to a certain extent.
Reyes S et al., Sleep Medicine 2019
Do Prevention Measures Work?
The results of the long-term study „Study on the Health of Children and Adolescents in Germany“ (KiGGS) by the Robert Koch Institute are now available for the years 2014–2017, following the baseline survey 2003–2006. For this study, the data of almost 3,600 children (3–17 years) were evaluated, including the BMI.
According to this, about 77% of children and adolescents in Germany are of normal weight. 15.6% of boys and 15.3% of girls suffer from overweight (including obesity), whereby the number of those affected increases with age. The proportions are similar to those in the years 2003 to 2006. Nevertheless, obesity before puberty is now somewhat less common.
Possibly this is a first sign of the effectiveness of obesity prevention for children in Germany. There are indications that a balanced diet and exercise options in childcare facilities have positive effects. However, it is still too early for a final evaluation.
Kurth B-M: Journal of Health Monitoring 2018
Is obesity transferable?
Obesity, cardiovascular diseases, cancer and diabetes are considered noncommunicable according to the WHO definition. The current study of an international research team now sees indications that many diseases classified as non-communicable may be transmitted from person to person via the microbiome.
According to this hypothesis, the microbial colonisation of humans with bacteria, fungi and viruses is essentially involved in this transmission path. According to the researchers, in animal experiments, laboratory animals kept together first of all resemble each other in their microbiome and finally in their external appearance.
It is so far certain that there is a connection between a disturbed microbiome and many diseases. Further studies will have to show whether the microbiome actually plays a role in the transmission of non-communicable diseases together with other influences, such as environmental conditions and genetic factors.
Finlay BB et al.: Science 2020
Overweightness primarily with disadvantaged children
Although the overall prevalence of overweight and obesity in children and adolescents has slightly decreased over the last decade, it has increased in the most disadvantaged regions. This is the conclusion of a new study based on data from more than one million children in Catalonia.
Childhood overweightness and obesity have increased in many countries with medium to high income populations. In Spain, around 41% of children aged 6–9 years were overweight and/or obese in 2015, the second highest prevalence rate in Europe. A new study is based on data from 1.1 million children and young people (2–17 years) in Catalonia between 2006 and 2016.
In general, the prevalence of overweight and obesity decreased slightly in both sexes and in all age groups. During the study period, the rate of overweight and obesity in the 6–11 year age group fell from 40% to 38% in girls and from 42% to 40% in boys.
However, rates rose in the most disadvantaged urban areas and among children of non-Spanish nationalities, which led to greater inequality. Between 2006 and 2016, the rate of obesity among girls aged 6 to 11 years fell by 15.8% in the districts with the highest socio-economic levels, but rose by 7.3% in the most disadvantaged areas. According to the authors, this data situation could be extrapolated to the whole of Spain as a whole.
Children from Latin America had the highest overweight / obesity rates, especially between the ages of 6 and 11 (56% boys / 50% girls) children of these nationalities were overweight and / or obese. Children of African and Asian nationalities had the greatest weight gain in the course of the study. The gradual adoption of western lifestyle and eating habits could explain these results, the authors said.
de Bont J et al., Jama Network Open. 2020
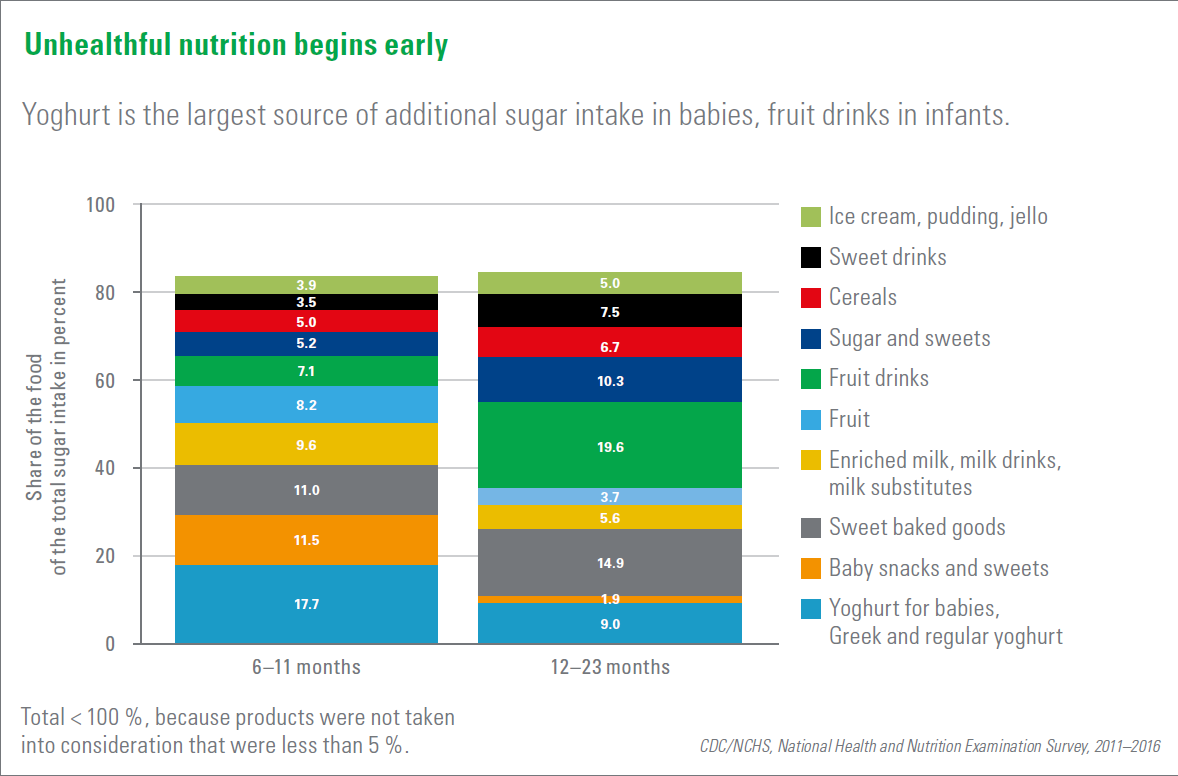
Sugar addiction
Almost one-third of US babies and 98 percent of infants consume added sugar in their daily diet – an increased risk of later obesity and other diseases.
Babies and toddlers have a natural preference for sweets. If this is further encouraged by increased sugar intake, eating habits develop that are difficult to break and lead to serious health problems. It is therefore twice as likely that six-year-olds will consume sugary drinks if they were given them in the first year of life. The connection with caries, asthma, overweight, high blood pressure and altered fatty acid composition in children has been proven in numerous nutritional studies.
US health organizations therefore recommend limiting daily sugar intake to 9 teaspoons for men and 6 teaspoons for women and girls over 2 years of age.
But the figures from the National Centers for Health Statistics are different: babies (6–10 months) already consume a whole teaspoonful of sugar per day infants (12–23 months) already consume the amount that would be permissible for an adult woman.
Above all, yoghurts and fruit drinks, but also sweets and sweetened baked goods are the main sources of excessive sugar consumption in children at a young age.
There were no differences according to gender, family income and head of household.
However, Asian toddlers consumed the least and black toddlers on the other hand consumed the m ost added sugar.
Herrick KA et al., Journal of the Academy of Nutrition and Dietetics
If you liked this post you may also like

