Allergy Prevention: Where are we now?
Allergy: Who is at risk?
It is already well established that family history raises a child’s risk of developing allergy later in life. Having one parent with allergy puts a child at 20-40% risk of developing an allergy; this risk roughly doubles if both parents have allergies. However, it should be noted that even among infants born to parents with no allergies, there is a 15% probability that an allergy will nonetheless develop. This thus raises the question of whether preventive strategies should also include even those who are considered to be at ‘low’ risk of developing allergy.
Reducing allergic response: Not all pHF are created equal
Heat techniques used in the manufacture of infant formula, such as sterilization, can alter the conformation of heat-labile proteins, resulting in the loss of conformational epitopes that elicit allergic response.[1] For example, heating of β-lactoglobulin – a whey protein – results in the formation of intermolecular disulfide bonds and altered protein folding, which subsequently makes β-lactoglobulin less allergenic. Linear epitopes, however, such as casein, are more heat stable than whey proteins. Enzymatic hydrolysis can reduce the allergenicity of caseins by breaking it down to smaller fragments that do not elicit immune response. An analysis of electrophoretic patterns of proteins from 12 hydrolysed milk formulas suggests, however, that manufacturers employ different processes of hydrolysis, and therefore, the resulting products may have varying allergenic potential.[2] These underscore that not all hydrolysed formulas are the same, and when considering a hydrolysed formula for allergy prevention, it is vital to choose one that has been proven or confirmed to have reduced allergenicity.
Allergy prevention with pHF: effective and cost-saving
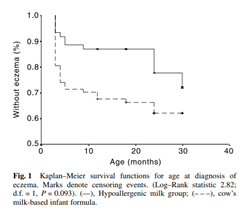
The GINI trial demonstrated that, compared with cow’s milk formula, the use of pHF (whey) for the first 4 months of life in nonexclusively breastfed infants at risk of allergy led to a lower incidence of allergic manifestations during childhood, and that the preventive effect persisted until the children were 11-15 years old.[i],[ii] Similarly, in Asian children, exclusive feeding of hypoallergenic milk formula in the first 4 months of life has been shown to confer a protective effect against the development of atopic dermatitis in the first 2 years of life [Fig. 1].[iii] Furthermore, a cost-effectiveness analysis has shown that early nutritional intervention with pHF (whey) as a replacement for standard cow milk formula in infants at risk was not only cost-effective but cost- saving compared to standard formula.[iv]
[1] Nowak-Wegrzyn A, Fiocchi A. Rare, medium, or well done? The effect of heating and food matrix on food protein allergenicity. Curr Opin Allergy Clin Immunol. 2009 Jun;9(3):234-7.
[2] Rosendal A, Barkholt V. Detection of potentially allergenic material in 12 hydrolyzed milk formulas. J Dairy Sci. 2000;83(10):2200-2210.
[3] von Berg A, Filipiak-Pittroff B, Kramer U, Link E, Bollrath C, Brockow I, et al. Preventive effect of hydrolyzed infant formulas persists until age 6 years: longterm results from the German Infant Nutritional Intervention Study (GINI). J Allergy Clin Immunol 2008;121:1442-7.
[4] von Berg A, Filipiak-Pittroff B, Schulz H, et al. Allergic manifestation 15 years after early intervention with hydrolyzed formulas--the GINI Study. Allergy. 2016;71(2):210-219.
[5] Chan YH, Shek LP, Aw M, Quak SH, Lee BW. Use of hypoallergenic formula in the prevention of atopic disease among Asian children. J Paediatr Child Health. 2002;38(1):84-88.
[6] Botteman M, Detzel P. Cost-effectiveness of partially hydrolyzed whey protein formula in the primary prevention of atopic dermatitis in high-risk urban infants in Southeast Asia. Ann Nutr Metab. 2015;66 Suppl 1:26-32.
