Nestlé Research A journey of research and innovation in the fight against allergies
Breast milk is perfectly composed to ensure that a child has the best start in life. Beyond the nutritional benefits, it has long been recognised that breastfeeding supports the infant’s protective immune response against common infections and reduces the risk of developing a range of non-communicable diseases, including allergic conditions. However, not all mothers are able to exclusively breastfeed their baby. For these mothers, access to formula with a nutrient and benefit profile inspired by breast milk is paramount. Food allergies present a major global health problem that, according to the World Allergy Organization, affects an estimated 240-550 million people worldwide. The risk of developing allergies is strongly influenced by early life experiences. Delivery mode, breastfeeding, early complementary feeding practices and environmental exposures are perhaps the most important risk modifiers for food allergy in infants and young children. Nestlé’s experience of more than 30 years in the field of paediatric allergy research has resulted in multiple clinically proven innovations which have helped to improve allergy prevention and management. This has also improved the quality of life of infants and their families. Nestlé Research is leading the way by connecting strong internal expertise with scientists and clinicians from world-leading academic institutions. In addition, Nestlé has successfully partnered with several start up companies who have developed novel technologies and products in the field of allergy.
NESTLÉ: A LEADER IN SPECIALISED NUTRITION AND ALLERGY
Over the past decades, the rising prevalence of allergies stimulated Nestlé Research to develop novel clinical solutions. Nestlé’s first partially hydrolysed formula was pioneered in 1987 in response to the surge in allergies observed since the 1960s. A hydrolysed formula contains cow’s milk proteins that have been partially or extensively reduced in size1.
Nestlé’s whey-based, partially hydrolysed formula (pHF-W) is the only clinically proven pHF-W with more than 20 publications demonstrating atopic dermatitis risk reduction2. The German Infant Nutritional Intervention Study (GINI), a large government funded study on the prevention of allergic diseases in children demonstrated that Nestlé’s pHF-W significantly reduced the risk of atopic dermatitis by up to 50% in the first year of life3. Participants of the GINI study have now been followed for more than 15 years4. This study has generated essential insights into the long-term health effects of pHF-W, extending from early infancy to adult life. While the findings from the GINI Study have been confirmed by numerous investigators, some studies have reported conflicting findings, as summarized in a recent meta-analysis5. In this context, it should be emphasized that each pHF-W is unique regarding its peptide profile (Fig. 1). This is likely to affect outcomes in clinical trials and a meta-analysis combining studies with different formulas and patient populations may miss beneficial effects of specific pHF-W products. When limiting the analysis to the Nestlé pHF-W, a peer-reviewed meta analysis confirmed the abovementioned risk reduction for atopic dermatitis2. Today, its efficacy has been demonstrated in infants from different countries, independent of family risk for allergic disease6. Nestlé Research continues to investigate the different mechanisms of action that could further enable this benefit. The use of pHF-W in at risk infants who are not breastfed has been shown to be a cost- effective risk reduction strategy in various health care settings, when compared to feeding with formula made with intact cow’s milk proteins7. Nestlé also has a long history of developing solutions for the nutritional management of cow’s milk protein allergy, with their first whey-based extensively hydrolysed formula (EHF) launched more than 30 years ago. Within the global Nestlé portfolio, there are two whey-based EHF. The first is a lactose-containing EHF suitable for the nutritional management of the majority of infants with cow’s milk protein allergy, and the second is a lactose-free EHF designed for milk-allergic infants with persistent gastrointestinal problems. Nestlé has used state-of-the-art technologies and science to develop these formulas, applying consistent and high-quality manufacturing8 in order to safeguard efficacy (Fig. 1). The manufacturing process is designed to minimise residual allergen content and to prevent external contamination with cow’s milk allergens. Moreover, all formulas have been tested in rigorous clinical trials to confirm their hypo allergenicity and ability to support normal infant growth9. More recently, Nestlé has added an amino 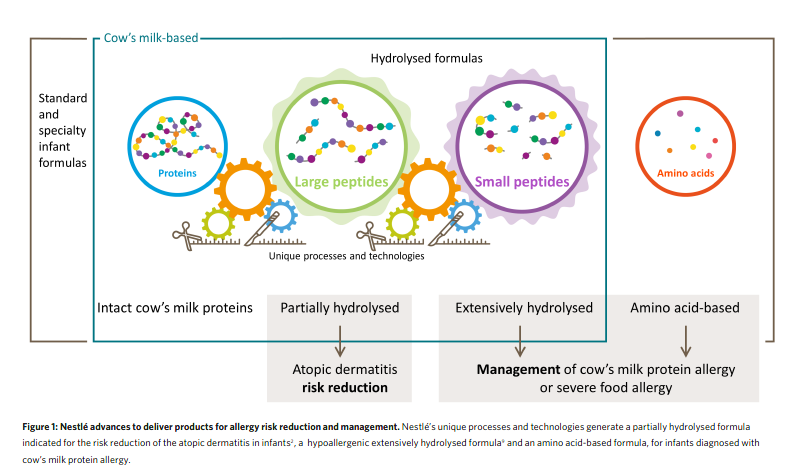
acid-based formula to its range of hypoallergenic formulas which is indicated for infants with severe symptoms of cow’s milk protein allergy and which, by design, is devoid of any residual cow’s milk proteins or peptides.
SHAPING THE DEVELOPING GUT MICROBIOME
The link between the commensal bacteria in our intestines (also known as the gut microbiome) and allergic diseases has been the focus of extensive research. Several studies have shown that infants delivered by Caesarean section or receiving antibiotics in early life have different gut microbiome compositions, and that these differences accompany a higher risk of developing allergies later in life10. This provides an opportunity to support the development of the microbiome in non-breastfed infants using probiotics (to deliver ‘good’ bacteria straight into the intestine), specific fibres or prebiotics (promoting the growth of beneficial bacteria). More recent studies investigating the relationship between microbiome, fibre and subsequent risk of allergies11 have also demonstrated a role for postbiotics (bacterial metabolites, such as short chain fatty acids) in reducing allergic airway inflammation and supporting immune modulation.
Postbiotics have since become an active field of study within Nestlé Research. Human milk oligosaccharides are complex carbohydrates which are a major component of breast milk. Research done on the developing gut microbiome has shown that human milk oligosaccharides in breast milk confer a range of clinical benefits, including a reduced risk of respiratory and gastrointestinal infections, as well as positive effects on overall gut health and even brain development12.
Nestlé has pioneered the addition of two human milk oligosaccharides in EHF and amino acid based formula9. HMO supplementation has been shown to reduce respiratory tract infections in healthy infants13. Furthermore, early preclinical evidence has linked human milk oligosaccharides with enhanced tolerance following allergen challenges. Human milk oligosaccharides may thus stimulate the immune system and
reduce the risk of food allergy12.
EARLY FOOD ALLERGEN INTRODUCTION
Emerging evidence suggests that exposure to dietary allergens in early life can reduce the risk of food allergies. The Learning Early About Peanut (LEAP) trial is a landmark investigation of early oral peanut exposure in high-risk infants14. In that study, introducing peanut into the diet of infants between 4 and 11 months of age reduced the prevalence of peanut allergy by 81%, compared to those who had strictly avoided peanut14. The emergence of baby food products containing one or multiple food allergens reflects a paradigm shift towards the early introduction of food allergens which is now part of many national and international prevention guidelines15.
Building on the research exploring early food allergen introduction, Nestlé has invested in Before Brands, a company that has pioneered products in this space. Their main product is designed to introduce the infant’s immune system to common food allergens in a baby-friendly format, as part of the complementary diet from 6 months of age16. Each serving contains a small amount of multiple food allergens which aim to prepare the infant’s immune system to tolerate a diverse diet. The use of this multi-allergen product reduces pressure on parents to introduce and maintain foods containing allergens in their infant’s diet, which in previous studies has been shown to be difficult to implement when following the allergy prevention guidelines17.
ORAL IMMUNOTHERAPY FOR PEANUT ALLERGY
Once a food allergy has been diagnosed, treatment relies on the strict dietary avoidance of the allergen. However, accidental exposure to food allergens, in particular to peanut, remains a significant problem. Oral immunotherapy is a novel treatment that aims to desensitize the patient (or prevent severe reactions) through gradual, repeated exposure to increasing amounts of a food allergen, such as peanut. Nestlé has recently acquired Aimmune Therapeutics, a California-based company that developed the only United States Food and Drug Administration approved oral immunotherapy treatment for peanut allergy. Several studies have shown that desensitization is achieved in the majority of peanut-allergic patients, allowing them to tolerate at least two peanuts without experiencing significant allergic symptoms after accidental exposure18. This new treatment approach has global significance, since more than 1.6 million children and young people in the United States experience peanut allergy, with around one in five of these people needing to seek emergency treatment each year.
ADVOCATING FOR SCIENCE-BASED ALLERGEN LABELLING
Nestlé pledges in favour of science-based allergen labelling that is clear and trustworthy for consumers, and harmonised so that allergic consumers find the level of information that is relevant to their health and safety. This mission must be continuously supported by scientific advances in the domain of analytical method development, validation and standardisation, as well as by best-in-class allergen risk assessment19. As a result, allergen labelling is the visible tip of the iceberg underpinned by strong research and development.
ADDRESSING UNMET NEEDS THROUGH CUTTING-EDGE SCIENCE
Nestlé develops science-based products to address specific unmet needs of allergic patients. This extends from the ‘classic’ domain of cow’s milk protein allergy in infants to peanut allergy in older children, and even environmental allergies, such as cat allergy. Innovation in the field of allergy can present in different ways, when considering Nestlé Purina research. Allergies to cats affect up to 1 in 5 adults worldwide. In many cases, this sadly leads to owners having to re-home their pets. After more than a decade of research, Nestlé Purina is the first and only company to develop a cat food that significantly reduces the major cat allergen Fel d 1 on cat hair and dander20. This innovative cat food is safe for the cat and has the potential to improve the quality of life for both cats and their owners by allowing closer interactions that strengthen the pet–human relationship20.
FUTURE INNOVATIONS
Nestlé is committed to continuing its scientific leadership in the allergy field and to identifying innovative solutions with scientifically proven safety and efficacy in the prevention, management and treatment of allergies. Efforts to further enhance existing products and deliver future innovations are based on continuous research, particularly in the field of breast milk composition and its physiological benefits. A recent key innovation of Nestlé Research is the development of formulas supplemented with human milk oligosaccharides which modulate the gut microbiome and may enhance
immune health12. Collaboration with academic groups to pursue the identification of infants at risk of developing allergies is another crucial element in Nestlé’s scientific strategy. Building on innovative solutions for allergy prevention and management, Nestlé is currently collaborating with several significant startup companies to accelerate the translation of early-stage discoveries into commercial products that will benefit allergic individuals and their families. More information is available on https://www.nestle.com/randd/ research-development-organization
REFERENCES
1. Fritsche, R., Pahud, J. J., Pecquet, S. & Pfeifer, A. J. Allergy Clin. Immunol. 100, 266–273 (1997).
2. Szajewska, H. & Horvath, A. World Allergy Organ. J. 10, 27 (2017).
3. von Berg, A. et al. J. Allergy Clin. Immunol. 111, 533–540 (2003).
4. von, Berg. A. et al. Allergy 71, 210–219 (2016).
5. Boyle, R. J. et al. BMJ 352, i974 (2016).
6. Sauser, J. et al. Int. Arch. Allergy Immunol. 177, 123–134 (2018).
7. Bhanegaonkar, A. et al. J. Pediatr. 166, 1145–1151 e1143 (2015).
8. Nutten, S., Schuh, S., Dutter, T., Heine, R. G. & Kuslys, M. Adv. Food Nutr. Res. 93, 147–204, (2020).
9. Nowak-Wegrzyn, A., Czerkies, L., Reyes, K., Collins, B. & Heine, R. G. Nutrients 11, 1447 (2019).
10. Tamburini, S., Shen, N., Wu, H. C. & Clemente, J. C. Nature Med. 22, 713–722 (2016).
11. Trompette, A. et al. Nature Med. 20, 159–166 (2014).
12. Vandenplas, Y. et al. Nutrients 10, 1161 (2018).
13. Puccio, G. et al. J. Pediatr. Gastroenterol. Nutr. 64, 624–631 (2017).
14. Du Toit, G. et al. N. Engl. J. Med. 372, 803–813 (2015).
15. Greer, F.R. et al. Pediatrics.143, e20190281 (2019).
16. Holl, J.L. et al. Pediatr. Allergy Immunol. 31, 418-420 (2020).
17. Perkin, M. R. et al. N. Engl. J. Med. 374, 1733–1743 (2016).
18. Vickery, B.P. et al. N Engl J Med. 379, 1991 (2018).
19. Yeung, J. & Robert, M. C. J. AOAC Int. 101, 70–76 (2018).
20. Satyaraj, E., Wedner, H. J. & Bousquet, J. Allergy 74 (Suppl. 107), 5–17 (2019).


