Multiple functional gastrointestinal disorders are frequent in formula-fed infants and decrease their quality of life
Multiple functional gastrointestinal disorders are frequent in formula-fed infants and decrease their quality of life
Authors: Marc Bellaiche, Raish Oozeer, Geraldine Gerardi-Temporel, Christophe Faure, Yvan Vandenplas
Keywords
Bloating, Colic, Intestinal gas, Quality of life, Regurgitation
Keynotes
- This multicentre French study evaluated functional gastrointestinal disorders (FGIDs) in infants up to six months of age to assess their incidence and impact.
- We found that 78% of the 2757 infants had multiple FGIDs: 63% had two and 15% had three or more.
- Multiple FGIDs were more likely to be associated with impaired weight gain, drug prescriptions and impaired quality of life than single FGIDs.
Abstract
Aim: This prospective study evaluated the incidence of functional gastrointestinal disorders (FGIDs) during infancy, on their own or combined with other symptoms.
Methods: We asked 273 French paediatricians with a specific interest in FGIDs to provide feedback on 2757 infants aged zero to six months from March 2013 to January 2014. Gastrointestinal health status was assessed by two questionnaires at inclusion and at a four-week follow-up visit. FGIDs were assessed according to the Rome III criteria and quality of life (QoL) was monitored.
Results: Combined FGIDs were diagnosed in 2145 (78%) infants: 63% with two disorders and 15% with three or more disorders. The most frequently combined FGIDs were gas/bloating and colic (28%), colic and regurgitation (17.0%) and gas/bloating and regurgitation (8%). Compared to infants with a single FGID, combined FGID were associated with lower body weight (4.63 vs 4.79 kg, p = 0.009), shorter breastfeeding duration (33 vs 43 days, p < 0.001), a decreased QoL score (5.9 vs 6.5, p < 0.001), more frequent drug prescriptions (25% vs 13%, p < 0.001) and significantly greater improvements in QoL scores after four weeks (p = 0.003).
Conclusion: Combined FGIDs were extremely common in infants up to six months of age and had a negative impact on breastfeeding, weight gain and QoL.
Introduction
Functional gastrointestinal disorders (FGIDs) are very common during the first year of life, and until 2016, they were defined using the Rome III criteria, which was the version used at the time of this study. They were then replaced by the Rome IV criteria. According to the Rome III criteria definitions, regurgitation has been estimated to affect as many as 30% infants worldwide, with infantile colic affecting 20% and functional constipation affecting 15% (1). FGIDs are a frequent reason for paediatric consultations in infants under four months of age, with around 23–28% of medical visits being motivated by gastrointestinal symptoms, mainly colic and regurgitation, according to French and Spanish studies (2,3). In some children, infantile colic may be associated with functional disorders later in life (4). Epidemiological data on FGIDs in infants are still scarce and heterogeneous (5), and we need information on FGIDs to be collected at the paediatric primary healthcare level. FGIDs are generally described as separate entities, but there is evidence that these disorders occur in different combinations (6–8). Alterations in gut microbiota have been linked with several gastrointestinal findings, such as delayed gut maturation, infantile colic (9) or excess gas and bloating (10), providing a clear indication that these symptoms are interconnected. Moreover, the impact of FGIDs and their severity on quality of life (QoL) is poorly described in the literature.
The aim of our prospective observational study, conducted in multiple primary paediatric outpatient practices, was to describe the incidence and impact of single or multiple FGIDs on infants during the first six months of life.
We also compared the epidemiological and clinical features of infants with single and multiple or combined FGIDs and assessed the impact on their QoL.
Methods
Study design and cohort
This prospective observational study was conducted with the help of 273 French paediatricians, who were all members of Approche des troubles Digestifs b enins Associ es du Nourrisson or ADAN Observatory, which was founded in 2013. The name of the organisation translates as infant minor-associated gastrointestinal disorders approach (11). Each paediatrician was asked to include the first ten infants who presented during a medical visit and complied with the inclusion criteria. The infants were recruited from March to December 2013 and the latest follow-up date was December 2014.
Patients eligible for inclusion were healthy, full-term infants aged zero to six months who were presenting for the first time with one or several minor digestive disorders. Infants also had to be exclusively formula-fed at the time of consultation, without any known allergy to cows’ milk protein.
Study format
Paediatricians performed a clinical examination and measured the infant’s quality of life (QoL) at the inclusion and follow-up visits. The timing of the follow-up visit was decided by the physician, but the recommended interval between visits was three to five weeks. During the inclusion visit, the paediatrician also collected data on the demographic and socio-economic characteristics of the parents, infant gender, birth weight, weight at inclusion in the study, birth order, breastfeeding and its duration, medication prescribed and additional investigations. At inclusion, the paediatricians were free to prescribe a range of infant formulas, including standard infant formula, which contained intact protein or partial hydrolysate, with or without prebiotics and/or probiotics, or fermented infant formula or goat’s milk infant formula. They could also prescribe infant formula classified as food for special medical purposes, such as thickened infant formula, cows’ milk and rice-based extensive hydrolysates, amino acid formulas or any medication. At the follow-up visit, the time interval between the two visits and the time it took to alleviate gastrointestinal symptoms were also recorded. Paediatricians recorded all information on completely anonymised documents, based on the information provided by the parents.
As this study collected data on the diagnosis and management of FGIDs by paediatricians and did not involve data collected from patients, the Ethical Board of the Robert Debre Hospital, which coordinated the study, did not consider it necessary to collect signed, informed consent from the parents.
Paediatricians evaluated the gastrointestinal symptoms at inclusion and follow-up visits and changes in gastrointestinal symptoms at follow-up versus inclusion by assessing the number of episodes of regurgitation, colic and/or constipation, as defined by Rome III criteria (12). They also included parental reports of gas/bloating, as this is a frequently reported gastrointestinal condition in medical consultations (13,14). Rome III criteria were applied to more than 90% of the infants, but these criteria could not be used to assess a small proportion of the subjects who were below three months of age. At the follow-up visit, the evaluation by the parents included a subjective assessment of the global change in FGID symptoms according to the following scale: failure, stationary status, moderate improvement, substantial improvement and full recovery. QoL was reported by the paediatricians at the inclusion and follow-up visits. Changes in QoL at the follow-up versus inclusion visits were evaluated by quantitative measurements of the infants’ QoL using the ten-grade Qualit e de vie du Nourrisson questionnaire, which is specifically designed to assess QoL in very young children (15) and ranges from one for very poor QoL to ten for excellent QoL. This tool was developed in France and validated by parents and paediatricians in a multicentre European study that involved 412 infants aged three months to three years (15). For this study, the questionnaire was adapted by omitting some of the items that were not applicable for the study age range of zero to six months.
Statistical analysis
Quantitative variables were given as mean values, standard deviations and ranges of the study population or group. Comparisons between the characteristics of infant presenting with single or multiple FGIDs were assessed using the chi-square test for discrete variables or the Wilcoxon test for nonpaired samples for continuous variables.
RESULTS
Participants
A total of 2757 infants were included: 602 (21.8%) infants presented with a single FGID and 2145 (77.8%) presented with more than one FGID. A further ten infants (0.36%) were excluded because of the absence of FGIDs at inclusion.
Patient demographic characteristics at inclusion
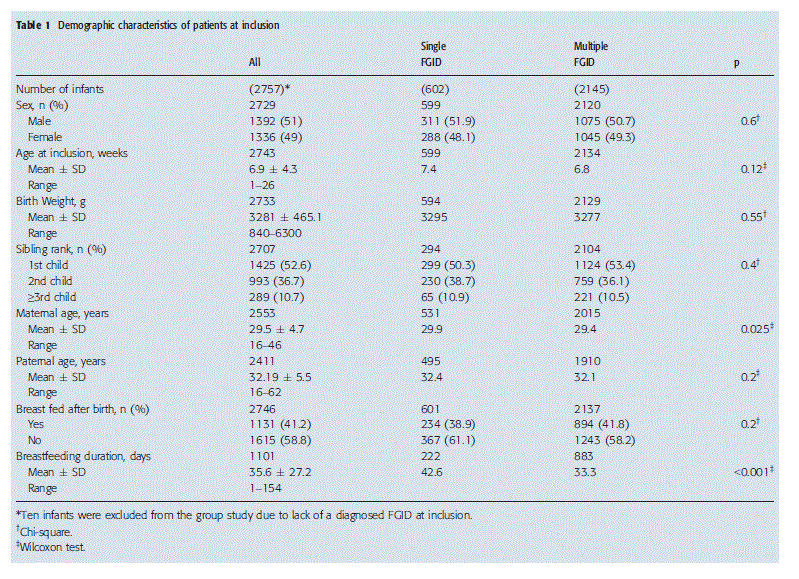
The demographic characteristics of the whole group and of the subgroups with one and multiple FGIDs are shown in Table 1. No statistically significant differences in gender, age, birth weight or birth order were observed between both subgroups (Table 1). On average, the mother was younger in the subgroup with a single FGID, whereas no difference in the age of the father was observed. The frequency of breastfeed- ing was similar in both groups. However, infants in the group with more than one FGID were breastfed for a shorter period of time than those presenting with only one FGID.
Patients’ clinical characteristics and QoL at inclusion
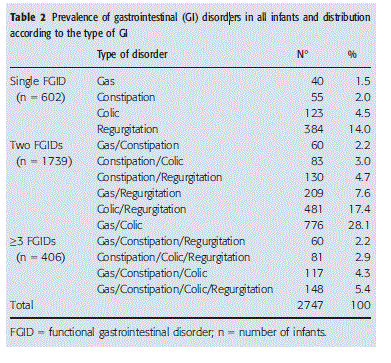
Globally, the most prominent GI symptoms diagnosed in our study population of 2757 infants were gas/bloating associated with colic, which was reported in 776 (28.1%) infants, followed by colic with regurgitation in 481 (17.4%), regurgitation in 384 (13.9%) and gas/bloating with regur-gitation in 209 (7.6%) (Table 2). Regurgitation was the most frequent symptom in the group with a single FGI and was present in 384/602 (63.8%), while constipation had the lowest incidence at 40/602 (6.6%).
In the group with more than one FGID, 1739 of 2757 (63.1%) infants were diagnosed with two FGIDs and 406 (14.7%) with three or more FGIDs (Table 2). The association of gas/bloating with colic was the most common combination in the group with multiple FGIDs (775/2142, 36.2%). Colic was the most frequently diagnosed concomitant symptom, occurring in association with other gastrointestinal disorders in 1686 of 2145 (78.6%) patients. In patients with three or more FGIDs, the combination of gas/ bloating with constipation, colic and regurgitation was the most frequent association of symptoms, occurring in 148 of 2145 (6.9%) infants (Table 2).
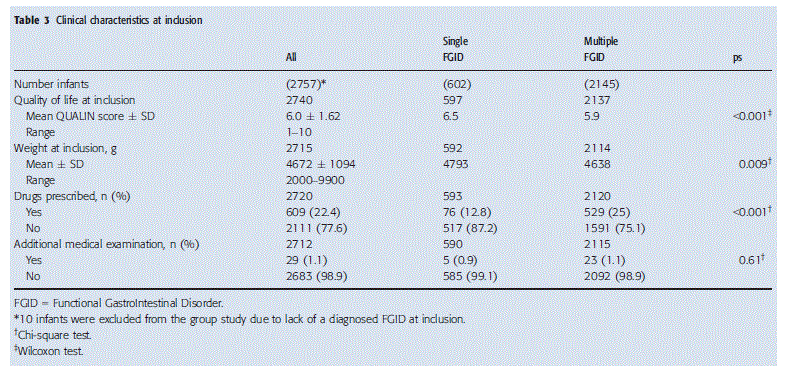
At inclusion, no difference in birth weight or age was observed between the two groups, but weight was significantly lower in infants with multiple FGIDs (Table 3). The presence of multiple FGIDs also had a negative impact on QoL (5.9 vs 6.5, p < 0.001) (Table 3). There was no difference in the demand for additional investigations between the groups (Table 3). However, medication was prescribed almost two times more often in the group with multiple FGIDs than in the group with only one FGID (25% vs 13%; p < 0.001). At inclusion, a change of infant formula, standard formula or food for special medical purposes, was recommended in 2728/2757 (98.9%) of all infants.
Changes in gastrointestinal symptoms at follow-up
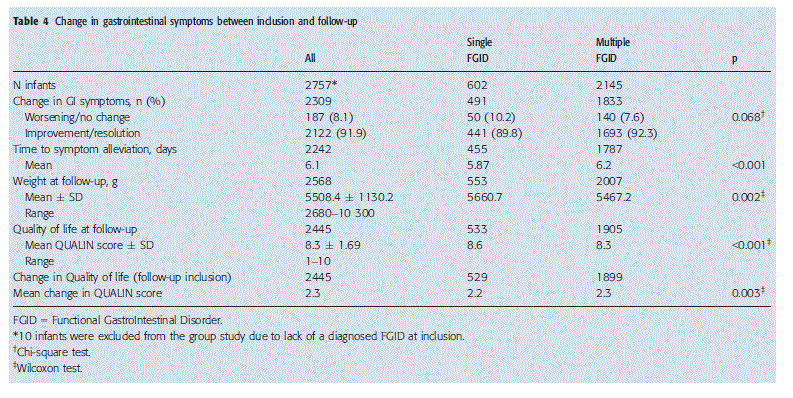
Between the inclusion and follow-up visits, 255/2757 (9.2%) of the infants were lost to follow-up for miscellaneous reasons. On average, follow-up visits were carried out 30.0 ( 10.7) days, (range 0–130) after inclusion, when the average age of the infants was 6.9 ( 4.4) weeks (range 1–26). There was no significant difference between the two groups for the timing of the follow-up visits. Subjective moderate or substantial improvements in symptoms were reported by the parents in 2122/2757 (77.0%) of cases (Table 4), with substantial improvements or resolution of symptoms in 1364/2757 (49.5%) of infants. Moderate or substantial improvements or complete resolution of symptoms were observed in 1406 of 1537 (91.5%) infants with colic, 1097 of 1199 (91.5%) with gas/bloating, 578of 621 (93.1%) with constipation and 1488 of 1695 (87.8%) with regurgitation (data not shown).
The number of patients showing moderate or substantial improvements or complete resolution of symptoms was similar in the two groups. However, the time to symptom alleviation was slower in patients with more than one FGID than in the group with only one FGID (Table 4).
Changes in other clinical characteristics and QoL at follow-up
The average weight of the infants in the whole group increased by 836 grams between inclusion and follow-up, and the average QoL score increased by 2.3 (Table 4). The significant difference in weight observed at inclusion between the two groups persisted during follow-up, with the weight at inclusion being lower in infants with multiple FGIDs (Table 4). Although patients in the group with multiple FGIDs showed a greater improvement in QoL between inclusion and follow-up (Table 4), the QoL scores in infants in the group with multiple FGIDs remained significantly lower than in the infants with a single FGID at follow-up (Table 4).
DISCUSSION
This study on infants with FGIDs diagnosed by 273 paediatricians across France revealed that multiple FGIDs occurred frequently during the first six months of life. The comparison with infants with a single FGID and those presenting with several FGIDs revealed that multiple FGIDs were associated with several demographic and clinical factors and that the presence of these associated disorders had a negative impact on their QoL. In this study population, 77.8% of patients were diagnosed with more than one FGID, with the combination of gas/bloating and colic being the most common association, occurring in 28.1% of the group. There were frequent associations between upper and lower FGIDs in these patients, with the combination of regurgitation with gas/bloating and colic or constipation being diagnosed in 820/2757 (29.7%) of the infants. Epidemiological data on the occurrence of multiple FGIDs are scarce. An online questionnaire-based survey of 2000 mothers in France found that gas/bloating occurred concurrently with regurgitation or constipation in nearly 40% and 30% of infants up to the age of 10 months, respectively (13). A prospective study of 2879 infants aged zero to six months, which was performed by 150 Italian paediatricians, found that multiple FGIDs were present in nearly 40% of the included infants (7). A smaller study of 320 infants in the United States found that multiple FGIDs occurred in 28.8% of infants and toddlers aged zero to three years (8). In our study, 14.7% of infants presented with three or more associated disorders. Similarly, 12.6% of infants in the Italian study were found to have three or more associated FGID (7), whereas the incidence in the US study was lower at 5.2% (8). The results of our study, and those of previous reports, demonstrate the high incidence of multiple FGIDs in early infancy and highlight the fact that associated FGIDs are a major concern for parents and a leading motivation for paediatric consultations.
To our knowledge, our study provides the first direct comparison of patient demographic and clinical factors between infants with single and multiple FGIDs. We found that multiple FGIDs were more frequent in infants born to younger mothers. Although the difference in age was statistically significant, the question only arises if a difference of just six months is clinically relevant. Infant discomfort and maternal anxiety are known to have an impact on infant well-being and gastrointestinal health (8,16,17). We also identified a significant association between shorter breastfeeding duration and multiple FGIDs. Gastrointestinal discomfort in infants often leads tochanges in feeding regimes, notably changes from breastfeeding to bottle feeding (7). Although we found no differences between the single and multiple FGIDs with regard to the frequency of breastfeeding at birth, the onset of multiple FGIDs was related to an earlier cessation of breastfeeding (33 vs 43 days). In the majority of cases, mothers had decided to stop breastfeeding before seeking medical advice, as an attempt to alleviate gastrointestinal discomfort. Bottle and breastfed infants have been shown to display significantly different arrays of gastrointestinal microbiome (18), and the reduced diversity and stability of gut microbiota have been implicated in the onset of infantile colic (9). Our findings could indicate that alterations in gut microbiota play a role in the development of multiple FGIDs.
The incidence of prescribed medication was relatively low, occurring in 22.4% of all infants and confirming findings from other studies (13). Prescribing medication for the treatment of FGIDs was two times higher in infants with multiple FGIDs than in those with a single symptom, presumably due to the increased perceived severity of symptoms in the group with multiple FGIDs. Similarly, Iacono et al. (7) found that among 93 infants requiring hospitalisation for FGIDs, most (62%) presented with multiple between two and five FGIDs.
Almost all (94.5%) of the paediatricians recommended changing infant milk formula to alleviate symptoms and this resulted in changes in 98.9% of the infants. This finding may illustrate the difference between the academic approach to managing FGID, which is reassurance and anticipatory guidance as a first and single approach, and the daily issues that general paediatricians face when parents expect them to do something. Nutritional advice has been shown to be often effective and has not been associated with any adverse effects, although it is often considered as unnecessary from a scientific point of view. However, the family paediatrician faces the problem of how to reassure anxious parents seeking medical help if no interventions are suggested.
Subjective improvements in symptoms were reported by parents in both groups between the inclusion and follow-up visits. This may be associated with the maturation of the gastrointestinal system and development of gut microbiota over the 30-day period between the visits (19). In this study, it is not possible to estimate the impact of formula changes, as they may have had mainly a placebo effect. What matters most is that the vast majority of parents perceived a clinically significant improvement in their child’s symptoms. Whether this improvement was real or just a perceived placebo effect is not very important, as reassurance is the recommended approach for FGIDs. The interventions of the paediatricians clearly provided reassurance. However, although there were no differences in the change in gastrointestinal symptoms between visits for the two groups, recovery was significantly slower in the group with multiple symptoms than in the group with a single FGID.
Low birth weight has previously been identified as a factor associated with gastrointestinal disorders (7), but we found no differences in birth weight between the two groups. We found that weight at inclusion was lower in infants with multiple FGIDs than in the infants presenting with one symptom. The weight deficit among the infants with multiple FGIDs persisted over the 30-day period. The difference in weight gain between both groups was statistically significant. If this difference of 200 g was clinically relevant is debatable, it suggests that feeding infants with multiple FGIDs are more difficult than those with a single symptom.
A limitation of our study was the fact that data on feeding regimens were not recorded for the period between birth and inclusion. However, it appears likely that the persistent weight differences observed between the two groups relate to a cumulative effect of multiple FGIDs on food intake, particularly, as we found that recovery was significantly slower in the group with multiple FGIDs. The study design allowed for different nutritional and pharmacological managements, but the impact of each one of these could not be analysed as they were not standardised.
Finally, a novel aspect of our study was the evaluation of QoL in infants with FGIDs using a multidimensional tool validated in several European countries and adapted for assessment of life quality in early infancy (15). FGIDs have previously been reported to have a negative impact on the QoL in toddlers aged two to three years (8). Our study shows that this negative impact was exacerbated by the presence of several associated FGID in infants aged zero to six months. Using the adapted French QoL questionnaire may also be a limitation of our study. However, until new tools are developed, there is currently no other alternative for assessing QoL in neonates. Despite this limitation, our findings highlight the need for paediatricians to assess the impact on QoL and, as a consequence, the need to propose adapted solutions for the management of FGIDs.
CONCLUSION
Multiple FGIDs were frequent in early infancy and were a key reason for consulting a paediatrician. The comparison between infants presenting with only one or multiple FGIDs allowed us to identify several demographic characteristics that occurred more frequently in infants with multiple symptoms. These included shorter duration of breastfeeding, as well as several clinical characteristics such as lower weight, an increased incidence of prescribed medication, slower recovery and impaired QoL. Paediatricians and parents should be made aware of these factors in infants presenting with multiple FGIDs and strategies to improve QoL need to be developed and implemented.

