The Link Between Early Diet and Food Allergy
Meeting Summary
During this symposium, leading experts in paediatric immunology discussed the influence of maternal and early infant diet in allergy prevention. This included evidence supporting the benefits of certain hydrolysed formulae and the role of early and diverse introduction of complementary feeding, including common allergenic foods. Monika Gappa showcased 20-year follow-up data from the German Infant Nutritional Intervention (GINI) study, showing that early nutritional intervention with extensively hydrolysed casein formula (eHF-C) or partially hydrolysed whey formula (pHF-W) exerts a preventive effect on both eczema and allergic airway manifestation that endures until adulthood. Carina Venter emphasised the importance of “letting the babies eat,” with early introduction of allergenic foods from between 4 and 6 months of age as part of a complementary diet, once the infant is developmentally ready to eat, recommended by the majority of medical and scientific societies worldwide, including the European Academy of Allergy and Clinical Immunology (EAACI). Kari Nadeau outlined evidence supporting early and regular introduction of diverse food allergens at low doses. Protein mixtures appear to show superior induction of an anti-allergenic state versus single foods, likely mediated by improvement in key biomarkers associated with sensitisation.
Early Interventions and Their Long-Lasting Effects on Childhood Allergies: The GINI Study 20-Year Results
Monika Gappa
Allergy development is a complex process and depends on numerous factors, including postnatal influences, that guide the immune system towards allergenicity or tolerance. Breastfeeding has a proven beneficial role, and some studies also support a preventative effect of partially hydrolysed infant formula on allergy development; however, controversies remain.1,2 This conflicting evidence stems from the different formulae used in different study protocols, with variability in the type and size of protein, as well as the hydrolysis process and site of hydrolysation, leading to differential effects on allergenicity and tolerance, explained Gappa.3 The GINI study set out to assess the differential influence of various hydrolysed infant formulae on the development of allergic disease compared to cow’s milk based formula (CMF) during
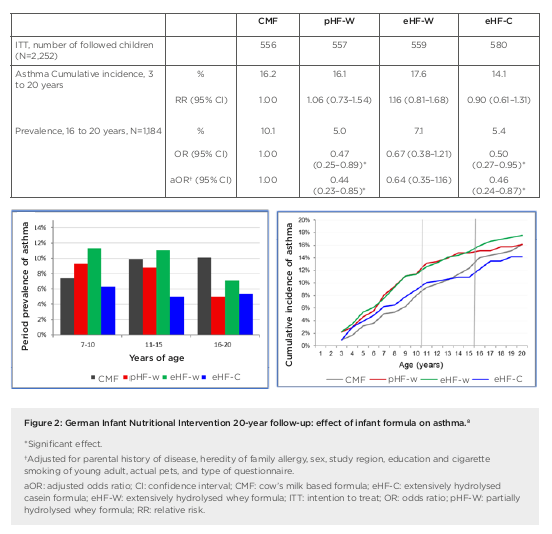
the first 4 months of life in at-risk children.4 Researchers enrolled a prospective birth cohort of 2,252 neonates (with atopic disease in at least one parent), who were randomised to receive either pHF-W, eHF-C, extensively hydrolysed whey formula (eHF-W), or CMF as reference exclusively until 4 months. Importantly, for data interpretation, this study remained double-blind until Year 3, noted Gappa. The hypothesis was that allergy risk could be reduced by lowering the allergen content in infant formula and inducing tolerance at the same time. There was no difference in weight gain between the treatment groups in GINI but original study findings showed a significant reduction in the risk of atopic dermatitis (AD) at age 3 years and 6 years for eHF-C and pHF-W, but not eHF-W, compared to CMF, explained Gappa.4,5 The risk from a positive family history of eczema was also attenuated by early nutritional intervention with eHF-C and pHF-W.6 At 15 years, further beneficial long-term effects of eHF-C and pHF-W on eczema and allergic rhinitis were recorded.7 These findings led to the conclusion that allergenicity and risk reduction depend not on basic protein or protein size, but most likely on allergenic epitopes resulting from specific hydrolysation processes. Overall, GINI represents the largest study in the field of allergy prevention with hydrolysates and the study with the longest follow-up to date. We now have data from 20 years plus the follow-up of the GINI study, noted Gappa, with data collected from 2016 to early 2018 during the field phase. The key challenge proved to be the change in main contact from parents to participants themselves. Nonetheless, over 70% of invitees took part in the follow-up, representing more than half of the original GINI interventional cohort. Twenty-year data from the GINI study revealed a persistent preventative effect of eHF-C and pHF-W on the period prevalence of AD, emphasised Gappa.8 The preventative effect of eHF-C was noted at all visits in the intention to treat and per protocol (PP) populations, while the preventative effect of pHF-W was more evident in PP. A significant beneficial effect of eHF-C and pHF-W was also seen on the cumulative incidence of eczema from birth to 20 years, with a 27% risk reduction for pHF-W (relative risk: 0.73; 95% confidence interval [CI]: 0.57–0.94) and 39% for eHF-C (relative risk: 0.61; 95% CI: 0.47–0.78) compared to CMF (Figure 1).8 Twenty-year follow-up of the GINI study has also revealed the impact of early dietary intervention on asthma, added Gappa. There was a significantly lower period prevalence of asthma in participants who received either pHF-W or male and female genders were combined.8 Odds ratios for the prevalence of asthma between 16 years to 20 years of age (n=1,184) were 0.47 (95% CI: 0.25–0.89) and 0.50 (95% CI: 0.27–0.95) for the pHF-W and eHF-C treatment groups, respectively (Figure 2).8
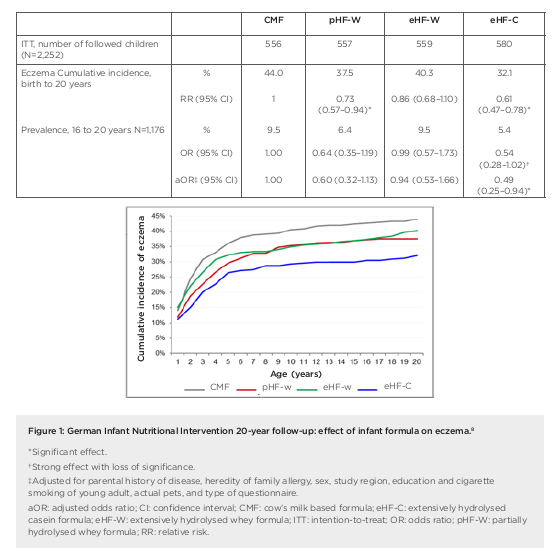
In summary, Gappa explained that 20-year follow-up data from the GINI study have highlighted the differential effect of infant formula on manifestation of atopic diseases. Both eHF-C and pHF-W conferred a strong and persistent reduction of AD and eczema from 3 to 20 years. A significant reduction in asthma period prevalence was also seen at age 20 years, with risk reduced by more than 50%. No such beneficial effects were noted with eHF-W. Moving forward, the GINI study is continuing to explore epigenomic, environmental, and lifestyle factors linked to allergy development in childhood. Emerging data will shed further light on the life-course epidemiology of allergic diseases. Overall, the GINI study has confirmed the hypothesis that early nutritional intervention with certain hydrolysed formulae, if exclusive breastfeeding is not feasible, has a preventive effect until adulthood for both eczema and allergic airway manifestation. Long-term data from the study showed a decrease in the prevalence of asthma after puberty and a
persistent beneficial effect on the incidence of eczema until adulthood with both eHF-C and pHF-W. Although breast feeding remains the gold standard, certain formulae may, therefore, represent an important part of multi-faceted interventions for allergy prevention, Gappa concluded.
How Do Maternal Diet and Infant Complementary Feeding Influence Food Allergy?
Carina Venter
Currently, there is limited evidence for the impact of maternal diet on allergy prevention and, based on current evidence, the greatest role is played by the introduction of complementary feeding and allergenic foods in infancy, explained Venter. EAACI guidelines advise against restricting consumption of potential food allergens during pregnancy or breastfeeding to prevent food allergy in infants and young children, based on randomised controlled trial evidence.9 A recent systematic review concluded that only prenatal supplementation with vitamin D may have beneficial effects for the prevention of asthma but no other offspring allergy outcomes.10 Studies looking at diet indices have also failed to show a consistent pattern of eating based on Healthy Eating Index (HEI), Diet Inflammatory Index (DII), or Mediterranean diet in pregnancy and subsequent allergy prevention in the infant. Although compelling data from animal models suggest that a high inflammatory diet may drive allergic outcomes, no association with asthma/wheeze by age 4 years was seen in a study from the USA looking at the inflammatory potential of diet in pregnancy and there was no effect on cord blood cytokine levels.11,12
More promising findings on the potential impact of maternal diet on offspring allergic diseases have come from analysis of the Healthy Start study, Colorado, USA. Results showed that a maternal diet rich in vegetables and yoghurt and with reduced intake of red meat, cold cereal, fried potatoes, rice and grains, and 100% fruit juice was associated with 50% reduced odds of AD 65% reduced odds of atopic wheeze, and 61% reduced odds of food allergy.13 So far, conflicting outcomes have been seen for the impact of overall maternal diet and pregnancy supplementation on allergy risk so we need to gather more data, summarised Venter. The only clear recommendation currently is that there is no need to avoid food allergens during pregnancy. Beyond maternal diet, the introduction of complementary feeding and allergenic foods in infants exerts by far the most important influence on food allergy risk. In answer to the question “Does it matter when we start weaning?” Venter explained that no randomised controlled trials have examined the age at introduction of complementary solid foods and the risk of food allergy, and noted that such trials would be difficult to conduct. However, summarising the evidence from three observational studies demonstrated, in a meta-analysis, no association between exposure to complementary solids at ≥4 months compared to <4 months of age and subsequent food allergy development.14 Both the intention-to-treat population of the LEAP study and the per protocol analysis of the EAT study also showed a significant reduction in food allergy prevalence when peanuts and eggs were introduced early, during the first year of life.15,16 Based on this cumulative evidence, updated EAACI guidelines on preventing the development of food allergy in infants and young children recommend introducing well-cooked, but not raw eggs or uncooked pasteurised egg, into the infant diet as part of complementary feeding.9 In populations where there is a high prevalence of peanut allergy, EAACI suggests introducing peanuts in an age-appropriate form as part of complementary feeding.9 According to available studies, EAACI concluded that the most effective age for egg and peanut introduction is between 4 months and 6 months of life.9 Venter also highlighted the important impact of diet diversity on allergy outcomes. The EAACI position paper identified that high diet diversity in the first year of life was significantly associated with reduced food allergy risk up to 6 years of age.17
This is supported by findings from the Isle of Wight study, which showed an 11% and 33% reduction in the probability of food allergies over the first 10 years of life when increasing numbers of food allergens (up to 15) were introduced over the first 6 and 12 months of infancy, respectively.18 Venter proposed that the mechanism underpinning this positive effect may lie in the impact of both overall food diversity and food allergen introduction on the infant microbiome. Studies have shown that the introduction of allergenic solids from age 3 months alongside breastfeeding was associated with differential dynamics of maturation of the gut microbial communities.19 Specifically, introduction of allergenic solids promoted a significant increase in Shannon diversity and representation of specific microbes, such as genera belonging to Proteobacteria and Firmicutes.19 The “disappointing” results from the intention-to- treat population of the EAT study may therefore be attributable to increased allergen diversity in both the early introduction and control group, suggested Venter. In summary, Venter stressed it was important to “let the babies eat.” Allergy prevention guidelines have evolved significantly over the past decade based on increasing evidence for the role of early infant diets in modulating future food allergy risk. At present, the only concrete advice to pregnant and breast-feeding women is that there is no need to avoid food allergens in their diet. Where infant feeding is concerned, early introduction of allergenic foods between 4 and 6 months as part of complementary diets, once the infant is developmentally ready, is recommended by the majority of medical and scientific societies worldwide and endorsed by EAACI guidelines. Diet diversity and allergen diversity are also recommended in infant complementary feeding as a key tool for the prevention of food allergies. We should aim to increase the variety of both foods and food allergens introduced, Venter concluded.
What Do Existing Food Allergy Prevention Studies Tell Us? What Does the Future Hold?
Kari Nadeau
Food allergy is a growing problem across the globe, highlighting the importance of finding preventative strategies to reduce food sensitisation risk and subsequent allergy. Avenues for prevention include early introduction of single and multiple foods in infants, explained Nadeau. Many studies, including the recent SPADE trial of early infant formula introduction to prevent cow’s milk allergy, have demonstrated a decreased risk for common allergies such as egg, peanut, and milk with early exposure to the allergenic food.21-23 The CHILD study pinpointed 4–6 months as the most beneficial time period for intervention; this optimal window for intervention was confirmed by a subsequent systematic review and meta-analysis.14,24 In the CHILD study, adjusted odds ratios for cow’s milk and egg sensitisation were just 0.29 and 0.97, respectively, when introduced before 6 months of age, compared to 3.69 and 1.89 if the allergen was avoided during the first year of life.24 Early is, therefore, better, emphasised Nadeau. There is also ample evidence to support a multi-allergen approach to reduce the risk of allergy, “by getting the immune system educated on different peptides at the same time,” explained Nadeau. The EAT study was a key multi-allergen trial. It showed a 67% reduction in allergy prevalence in the PP group, who were given one or more allergenic foods.15 Retrospective epidemiological studies, such as PASTURE, have also demonstrated that greater food diversity in infancy/toddlerhood lower the risk of food allergy “quite dramatically,” and also have a beneficial impact on other atopic conditions.25,26
A multivariable analysis by Venter et al.18 confirmed a 96% reduction in food allergies over the first 10 years of life in infants who consumed eight allergenic foods by 12 months of age. “Diversification of diet early and often is therefore very important,” stressed Nadeau, “and this has been shown time and time again by many independent groups.” The I’M EATING STUDY was another key multi-allergen trial where infants <12 months old were randomised to an active group (1 tablespoon a day of a powdered food supplement containing 16 protein allergens) or a placebo group.27 The daily multi-allergen food supplement was found to be safe with good tolerability. The study found no increased risk of rash/eczema. Further, during follow up, none of the participants were diagnosed with food allergy.27 Nadeau added that families in this study felt excited about being able to diversify their diet, start consuming table foods early, and not “live in fear of allergies.” Although the EAT study found that early intervention with allergenic foods was beneficial in reducing food allergy, the higher serving sizes used in this study are thought to increase non-compliance, cause early satiety, reduce breast milk consumption, and/or lead to more complicated preparations of food than needed. Therefore, serving sizes in the context of caloric intake recommendations for infants, as provided by the U.S. Department of Agriculture (USDA), should be an important factor to consider during early intervention of multiple allergens. Caloric intake limitations are provided by the USDA to prevent diabetes, obesity, and other diseases. Fortunately, studies such as the PETIT study using egg powder have shown that high serving sizes may not be needed to prevent food allergies. The PETIT study found that egg allergy prevention can be achieved with much lower servings of food.28 Biomarkers can also reveal important insights into food prevention studies. In vitro exploratory data using 54 well-characterised mono- and multi-allergic patient samples showed that exposure to a complex mixture of proteins induced an anti-allergenic state, which was superior to single or double protein preparations. The mixture of allergenic proteins showed consistently superior improvements in biomarkers associated with allergic sensitisation, such as sIgE, sIgG4, and T cell diversity index. Nadeau and others have shown in prior publications that activation of dendritic cells increases IL10 and boosts IgG4 isotype switching capacity, leading to a decrease in specific IgE production by B cells. To explore this effect in vivo, a 1-year feeding study was conducted at Stanford using a 10-food protein mixture. It prospectively evaluated the optimal dose for food allergy prevention as determined by a gold standard oral food challenge (OFC).29 A total of 180 infants, 50% from high-risk families, were randomised to one of the following groups: control, single food protein, double food protein, a low serving of mixture (300 mg/day), a medium serving of mixture (900 mg/day), and a high serving of mixture (3,000 mg/day). OFC with a mixture of 15 different foods was carried out after 2–4 years. In the intention to treat population, 48% of the control group had an objective allergic reaction and failed the OFC. In the single milk, egg, or peanut protein early intervention groups, positive allergic reactivity was observed in 40–50% of participants, compared to approximately 30% in those that received doubles.29 In contrast, infants who received a mixture showed statistically significantly lower reactivity, 7% in the high and medium serving mixture groups and 0% in those who received the lowest serving mixture group. Although regular to every other day feeding was optimal, those who stopped and started again weeks later were not at higher risk for developing food allergies. There was no increase in eczema, even in the high-risk infant, indicating that the mixture was safe. Biomarkers from this study showed greater increases in peanut-specific and milk-specific IgG4/IgE ratios with mixtures versus double or single protein alone.29 Failure to introduce foods early can cause harm, cautioned Nadeau, by impairing immune.
Insights from primary prevention trials have shown that longitudinal egg-specific regulatory B and T cell development was improved when eggs were introduced early in both high- and low-risk infants.30 Similar findings were seen in a randomised trial of peanut consumption in at-risk infants where every month of peanut avoidance was associated with an increase in peanut wheal size and peanut-specific IgE.16 Based on the evidence presented, Nadeau outlined five key recommendations on food allergy prevention: 1) There is no reason to test infants for allergies before introducing diverse foods, and babies with normal, dry, and any degree of eczema can start diversifying foods early and often. 2) Eczema or AD is not worsened by introducing diverse foods, in fact, it typically gets better, said Nadeau. 3) Larger serving sizes do not seem to be needed, but daily (not weekly), small servings seem optimal and age-appropriate without exceeding calorie intake recommendations. 4) Data show that it is safe to introduce diverse foods and that stopping/ starting particular foods does not increase food allergy. 5) Introducing small servings of diverse foods can be done together with breastfeeding or formula feeding and is consistent with World Health Organization (WHO) guidelines, Nadeau concluded.
Looking to the future, further studies exploring the prevention of food allergy are needed. It is important to conduct food challenges and mechanistic evaluations during immunotherapy protocols. Biomarker assays show promise for monitoring treatment response. Overall, Nadeau reiterated that prevention of food allergy is possible with early and regular introduction of small servings of diverse foods.
Question and Answer
The symposium concluded with an interactive question and answer session led by Mahony. In response to a question from the audience, Gappa confirmed that use of hydrolysed formulae for the prevention of allergy could be recommended based on the data presented, but that it was important to consider only those specific formula that have shown a beneficial effect on allergy development in clinical studies. Mahony described it as “amazing” that choice of formula early in life can influence a disease that happens 20 years later. The panel speculated that the reason why impact on asthma was not seen until puberty in the GINI study may have been due to delays/shortcomings in diagnosis. Asked about the contribution of host genetics to allergenic response, Venter described this as an “interesting” area. Preliminary data have pinpointed potential epigenetic markers associated with IgE-mediated food allergy, but bigger cohort studies are needed to look at these complex underlying factors in more detail. Mahony added that secretor versus non-secretor mutations that determine levels of fucosylated human milk oligosaccharides in breastmilk are already known to exert a modulatory effect on the infant microbiome population. In response to an audience query, Nadeau confirmed that there is “absolutely no need” for IgE testing before starting solid foods, even in infants with severe uncontrolled AD; rather, it is “imperative” to start feeding early, she stressed. Studies have shown that early introduction of food counters the allergic processes leading to AD and eczema because 70% of the body’s immune system is centered in the gut. Mahony noted that a wide body of evidence now supports the early window of opportunity during the first 6 months of life as being “incredibly important.” Nadeau suggested that, before crawling and walking, early exposure to food is likely key to priming the developing immune system. Between 4 months and 6 months also coincides with the nadir of maternal antibodies, which means the infant’s own memory immune system is “ramping up” to protect itself, making it the “sweet spot” for exposures, said Nadeau.
On the issue of guidelines, Gappa acknowledged the difficulties in reconciling the available evidence given clear methodological shortcomings with some studies. She stressed that it was important for guidelines on allergy prevention to differentiate those with good quality evidence for a preventive effect and not just any hypoallergenic formula. The allergenicity of a specific formula will also vary according to the process of manufacture so in vivo testing and, ideally, confirmatory infant studies are important. Not all hydrolysed whey-based formulae will exert the same effect, said Gappa, as the hydrolysation process itself is very specific. Venter explained that it was important not to categorise foods, notably dairy, as “bad” when considering diet indices. Better tools are needed to clearly define food patterns and diet indices, she noted, with accurate statistical analysis that is able to take complex factors into account. Mahony asked whether early introduction of allergens should focus exclusively on proteins or consider whole foods. Nadeau responded that the entire “instruction sheet” for the immune system on allergy prevention is protein based. However, adequate representation of primary, secondary, and tertiary protein structures is essential because these conformational epitopes ensure complete immune system education. While carbohydrates and fats are not required for allergy prevention exposures, they remain nutritionally important. The whole paradigm of early dietary intervention is to remove the fear of food allergies and facilitate transition to table foods that will enrich the child’s diet to “make every spoonful and bite count nutritionally,” Nadeau concluded.
References
1. Sauser J et al. Partially hydrolyzed whey infant formula: literature review on effects on growth and the risk of developing atopic dermatitis in infants from the general population. Int Arch Allergy Immunol. 2018;177(2):123-34.
2. Boyle R et al. Hydrolysed formula and risk of allergic or autoimmune disease: systematic review and meta- analysis. BMJ. 2016;352:i974.
3. Bourdeau T et al. Peptide characterization and functional stability of a partially hydrolyzed whey-based formula over time. Nutrients. 2021;13(9):3011.
4. Rhezak P et al. Short- and long-term effects of feeding hydrolyzed protein infant formulas on growth at < or = 6 y of age: results from the German Infant Nutritional Intervention Study. Am J Clin Nutr. 2009;89(6):1846-56.
5. von Berg A et al.; GINIplus study group. Preventive effect of hydrolyzed infant formulas persists until age 6 years: long-term results from the German Infant Nutritional
Intervention Study (GINI). J Allergy Clin Immunol. 2008;121(6):1442-7.
6. von Berg A et al.; GINIplus study group. Impact of early feeding on childhood eczema: development after nutritional intervention compared with the natural course - the GINIplus study up to the age of 6 years. Clin Exp Allergy. 2010;40(4):627-36.
7. von Berg A et al.; GINIplus study group. Allergic manifestation 15 years after early intervention with hydrolyzed formulas – the GINI study. Allergy. 2016;71(2):210-9.
8. Gappa M et al. Long-term effects of hydrolyzed formulae on atopic diseases in the GINI study. Allergy. 2021;76(6):1903-7.
9. Halken S et al. EAACI guideline: preventing the development of food allergy in infants and young children (2020 update). Pediatr Allergy Immunol. 2021;32(5):843-85.
10. Venter C et al. Dietary factors during pregnancy and atopic outcomes in children: a systematic review from the European Academy of Allergy and Clinical Immunology. Pediatr Allergy Immunol. 2020;31(8):889-912.
11. Venter C et al. Examining associations between dietary inflammatory index in pregnancy, pro-inflammatory cytokine and chemokine levels at birth and off-spring asthma and/or wheeze by age 4 years. J Acad Nutr Diet. 2021;121(10):2003-2012.e3.
12. Venter C et al. Advanced glycation end product intake during pregnancy and offspring allergy outcomes: a prospective cohort study. Clin Exp Allergy. 2021;51(11):1459-70.
13. Venter C et al. The maternal diet index in pregnancy is associated with off-spring allergic diseases: the Healthy Start study. Allergy. 2021;DOI:10.1111/all.14949.
14. Burgess JA et al. Age at introduction to complementary solid food and food allergy and sensitization: a systematic review and meta-analysis. Clin Exp Al-lergy. 2019;49(6):754-69.
15. Perkin MR et al. Randomized trial of introduction of allergenic foods in breast-fed infants. N Engl J Med. 2016;374(18):1733-43.
16. Du Toit G et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372(9):803-13.
17. Venter C et al. EACCI position paper on diet diversity in pregnancy, infancy and childhood: novel concepts and implications for studies in allergy and asthma. Allergy. 2020;75(3):497-523.
18. Venter C et al. Different measures of diet diversity during infancy and the association with childhood food allergy in a UK birth cohort study. J Allergy Clin Immunol Pract. 2020;8(6):2017-2026.
19. Marrs T et al. Gut microbiota development during infancy: impact of introducing allergenic foods. J Allergy Clin Immunol. 2021;147(2):613- 21.
20. Osborne NJ et al. Prevalence of challenge-proven IgE-mediated food allergy using population- based sampling and predetermined challenge criteria in infants. J Allergy Clin Immunol. 2011;127(3):668-76.
21. Ierodiakonou D et al. Timing of allergenic food introduction to the infant diet and risk of allergic or autoimmune disease: a systematic review and meta-analysis. JAMA. 2016;316(11):1181-92.
22. Peters RL et al. Early exposure to cow’s milk protein is associated with a reduced risk of cow’s milk allergic outcomes. J Allergy Clin Immunol Pract. 2019;7(2):462-70.
23. Sakihara T et al. Randomized trial of early infant formula introduction to prevent cow's milk allergy. J Allergy Clin Immunol. 2021;147(1):224-32.
24. Tran MM et al. Timing of food introduction and development of food sensitization in a prospective birth cohort. Pediatr Allergy Immunol. 2017;28(5):471-7.
25. Roduit C et al.; PASTURE study group. Increased food diversity in the first year of life is inversely associated with allergic diseases. J Allergy Clin Immunol. 2014;133(4):1056-64.
26. Roduit C et al.; Protection Against Allergy–Study in Rural Environments study group. Development of atopic dermatitis according to age of onset and association with early-life exposures. J Allergy Clin Immunol. 2012;130(1):130-6.e5.
27. Holl JL et al. A randomized trial of the acceptability of a daily multi-allergen food supplement for infants. Pediatr Allergy Immunol. 2020;31(4):418-20.
28. Natsume O et al.; PETIT Study Team. Two-step egg introduction for prevention of egg allergy in high-risk infants with eczema (PETIT): a randomised, double-blind,
placebo-controlled trial. Lancet. 2017;389(10066):276-86.
29. Quake AZ et al. Early introduction of multi-allergen mixture for prevention of food allergy. Preprints. 2021;DOI:10.20944/ preprints202112.0257.v1. [Preprint].
30. Lai C et al. Longitudinal egg-specific regulatory T- and B-cell development: insights from the primary prevention clinical trials examining the timing of egg introduction. Allergy. 2021;76(5):1385-97.

