Growth and Immunity – Early Nutrition Influence
Welcome Note
One crucial impact for the so important first 1000 days in life is nutrition. Because we know now from the research, this can really have a long-term impact on health. We have a good example. We know actually what is the best nutrition after birth – if you breastfeed your baby exclusively for the first six months.
For the benefits of mother’s milk there is one important factor: Human oligosaccharides. Besides lactose and fat, human milk oligosaccharides (HMOs) belong to the third most abundant group of milk components. Currently they are the great nutritional gap between human milk and infant formula. Although we know HMO’s and their beneficial effects for a rather long time, HMOs are a very exciting and a rather new topic for infant’s nutrition.
We have done more than 30 years of research in this field but we are still in the beginning. We have clearly understood that it is an important factor in breast- milk and have a unique impact in the microbiome. But we are at the beginning of a new era. Today the large scale production of some HMOs is possible, and clinical studies are currently performed. It is like a step on the moon, HMOs are really one of the hottest topics in infant nutrition.
Even the first studies on formula supplementation with specific human milk oligosaccharides are performed. How do that influence the microbiome of infants? Are there benefits similar to breastfed babies? The progress is very exciting, but there a lot of questions to solve.
First we have to understand them, second is to be able to produce them and then we have to provide safety studies to infant formulas. The EFSA and the FDA had improved the safety of specific HMOs we are using now. Now it is important to let the message get out.
We need your dialogue, we need your questions, that is why I really invite you for the discussion rounds. After each session, we would like to invite you to a podium discussion to exchange ideas and voice any specific concerns.
Welcome and thank you for participating!
PART 1: NUTRITION AND MICROBIOM
Microbiome research – Current success and further challenges
The speaker: Bernard Berger, Switzerland
Barely more than a decade ago, very few of us would have bet that the research on the human microbiome would develop into a booming research field of complex systems involving the host and its colonizing microbial community.
At present, the microbiome is recognized as an organ that significantly contributes to human biology and development, even able to influence mood and behavior. It is a very special organ, since it evolves throughout life and we can modify it.
The microbiome embraces all the microorganisms inside us or on our skin (bacteria, fungi and viruses) and the metabolites that they produce. The term “microbiota” is relatively similar but is generally restricted to bacteria. In our gut, there are 10 x more bacterial cells than our body cells, more than 1000 bacterial species harboring 150 x more genes than in our genome. In adult, the gut microbiome is not randomly assembled. Three favored combinations of bacteria have been observed and defined as the enterotypes. They are driven by bacteria belonging to Bacteroides, Prevotella or Ruminococcaceae/Lachnospiraceae. It seems that the enterotypes are linked to long- term diets. But more interesting, there is an inter-relationship between the microbiome and human health, beyond the gut. More and more diseases are associated with dysbiosis, a microbial imbalance in the gut ecosystem. Studies in preclinical models have shown that these dysbioses can be transferred to germ free animals through fecal transplant, with concomitant transfer of at least part of the phenotype of the diseases.
We also see a very impressive development of in vitro models of the gut interface, mimicking the host-microbes interactions and allowing the selective sampling of the different components playing a role in this ecosystem.
The research on the human microbiome is a scientific breakthrough of the last decade. Al- though this research field looks very promising, we are still far from having a sufficient under- standing of this new organ to translate all these scientific successes into standard for nutrition and therapeutics.
The early life microbiome
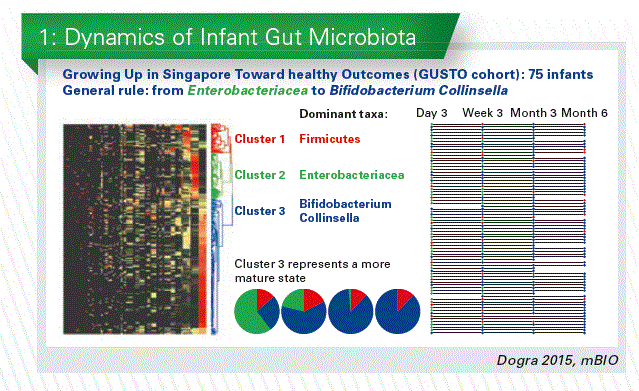
Gut microbial colonization essentially starts at birth and this early and progressive exposure to microorganisms is advocated to exert a key impact on the neonate and infant health that may be lasting later in life. The early life gut micro-biome is far less complex than the adult one and – importantly – is very reactive to environmental and nutritional stimuli. In collaboration with the Epigen consortium, we performed in Singapore an analysis of the microbiota of infants at day 3, week 3, month 3 and month 6 (Dogra et al. 2015, mBio). The taxonomic profiles of all the samples were compared and clustered by similar- ity. Three clusters of profiles were defined, dominated by Firmicutes, Enterobacteriacea or Bifido- bacterium/Collinsella. We observed a progressive increase in the proportion of infants belonging to the Bifidobacterium-dominated community type, suggesting a more mature state. (Chart 1).
Studies in other countries made similar observations, showing an evolution of the infant microbiota through bacterial community types, which may be more important than the abundance of single bacterial taxa. The establishment of the gut microbiota in infancy is a dynamic process, whose rate may be important.
The proportion of Bifidobacterium in early life gut microbiota, as reported in the literature, seems to be country-dependent. However, three papers describing the same cohort in Canada showed very different results (Chart 2). This large and in- consistent variability between studies raises the question of the differences linked to the applied analytical methodologies, a well-documented problem. To our knowledge only one study documented differences in the proportion of Bifido- bacterium between countries (Finland, Estonia,
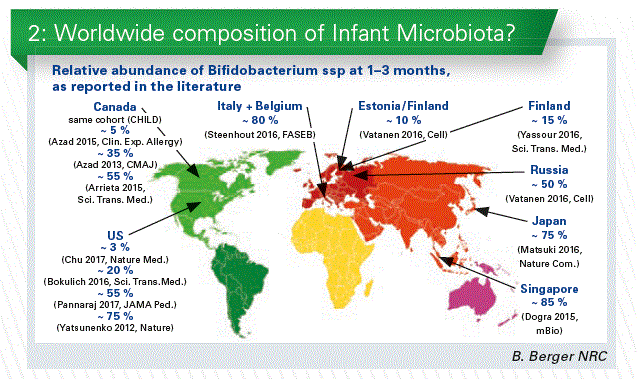
and Russia) using the same methodology. We may have geographical differences, but to describe the infant gut microbiota around the world, a standardized methodology would be necessary.
The origin of the infant microbiota
Many factors seem to influence the microbiota in early life. However, here the question is not what is influencing, but what is seeding the microbiota. The origin of the infant microbiota is a relatively difficult topic since the sources of microorganisms discussed in the literature are numerous: the placenta, the rectum of the mother, the vagina, the skin, the breast-milk, the family and siblings, the pets, and more generally the environment. The rectum as source of the infant gut microbiota is formally established. The breast-milk seems to contribute, but the origin of its associated microbiota is not clear. Retrograde flux from the mouth of the infant to the mother breast is complicating the question of the origin. Nevertheless, the breast milk may play a role as a reservoir for continuous seeding of the infant microbiota. The existence of a placental microbiota is controversial and we encourage the audience to form its own opinion by reading a re- cent review [A critical assessment of the “sterile womb” and “in utero colonization” hypotheses: implications for research of the pioneer infant microbiome (Perez-Mundoz et al., 2017)].
The vagina – is it really seeding? It seems obvious since differences of microbiota were observed between C-section and vaginal delivery infants. However, these differences do not imply a large contribution of the vagina in term of quantity of transferred bacteria. In a recent study in collaboration with the Epigen consortium, we analyzed the microbiota of infant stools at day 3, maternal stools and vagina samples (Sakwinska et al. 2017 Beneficial Microbes), and we looked for the species shared between the three ecological niches. The contribution of the vaginal microbiota to the infant stool microbiota is small, irrespective to the mode of delivery. These results are consistent with recent publications. The contribution of the maternal gut microbiota to the infant gut microbiota is much more important than the vaginal microbiota. We should scrutinize and re-evaluate the dogma.
This observation does not rule out the vaginal microbiota as an important factor shaping the infant colonization. For example, the lactobacilli (a major component of the vaginal microbiota) are observed in low amounts in the infant stool microbiota but have a strong effect on the overall composition of the infant gut microbiota (the keystone species concept, as defined in ecology). Consistent with this idea, the results of a nutritional intervention in bottle-fed babies showed that a probiotic Lactobacillius reuteri strain could correct the gut microbiota dysbiosis observed in C-section delivered infants (Garcia Rodenas et al. 2016 JPGN). Likely, the lactobacilli created an environment favorable to the growth of bifidobacteria and influenced the whole microbiota composition.
Human Milk Oligosaccharides
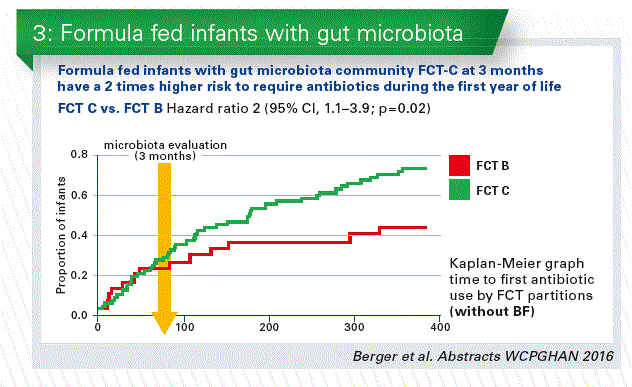
- We can learn from the human breast milk. The higher abundance of human milk oligosaccharides (HMOs) is one of the major differences between standard infant formula milk and human breast milk. The classical prebiotics (FOS, GOS), which are sometimes added to infant formulae, have much more simple structures compared to HMOs. We conducted the first safety clinical trial in infants with 2 HMOs, 2’FL+LNnT (Puccio et al. 2017 JPGN). The results of the clinical parameters are discussed in another talk of this session. We added a reference breastfed (BF) arm, and analyzed the stool microbiota. At 3 months, microbiota composition in the HMOs group appeared closer to BF than control by microbiota alpha (within sample) and beta (between samples) diversity analyses. Regardless of the feeding group, we identified three fecal community types (FCT) in infant stools at 3 months. Both FCT B and C were dominated by Bifidobacterium spp, but with a higher abundance in B, whereas FCT A was Enterobacteriaceae and Lachnospiraceae dominated. HMOs supplementation decreased the number of infants with formula specific FCT C and increased those with BF specific FCT B. Formula fed infants with FCT C at 3 months have a 2 times higher risk to require antibiotics during the first year compared to FCT B (Chart 3 + 4).
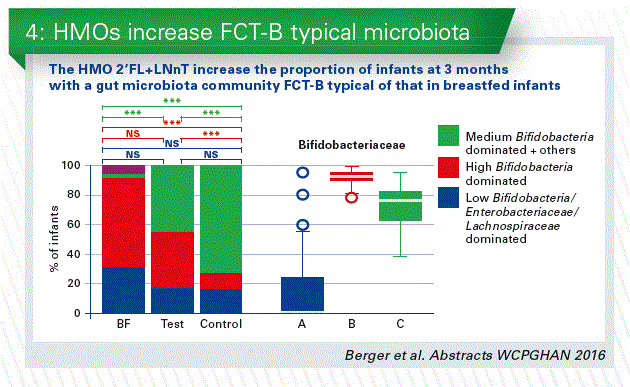
Conclusion
First clinical data show that formula with 2’FL+LNnT promotes a fecal community type seen in breastfed infants.
The likelihood of antibiotic usage is likely associated to the fecal community types.
After these first promising health outcomes, further studies are needed to establish efficacy.
Influence of breastfeeding on microbiota and its health benefits
The speaker: Maria Carmen Collado, Spain
Maternal microbiota is being recognized as one of the essential factors determining maternal-child health outcomes, which would also be affected by perinatal factors.
Microbiome colonization steps has an important effect in nutritional, microbiological, met- abolic and immunological programming.
Different studies had been shown that the infant microbiome is changing a lot during the first year of life.
The development of the human gut microbiome is a complex and step-wise process. There are some evidence that maternal microbiome plays an important role during gestation. At birth we already know the massive exposure of the microbes. The mode of delivery influences the type of microbial exposition. At birth maternal microbes contact with the infant through delivery and then is supported by breastfeeding. Of course genetics, environmental factors and diet may influence the process of microbiota (Chart 1).
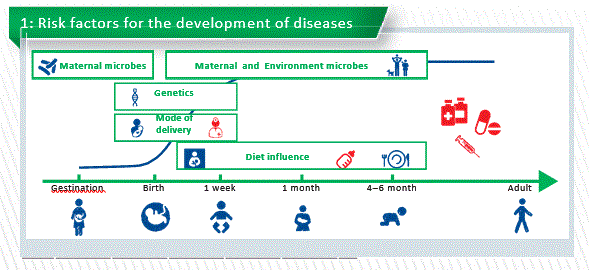
Factors influencing microbiota
- Mode of delivery
- Vaginally delivered infants acquired bacterial communities resembling their own mother’s vaginal microbiota, dominated by Lactobacillus, Prevotella, or Sneathia spp. Higher bacterial diversity is also present in vaginally delivered infants.
- C-section infants harbored bacterial communities similar to those found on the skin and oral surface, dominated by Staphylococcus, Corynebacterium,and Propionibacterium spp. Lower diversity.
Mode of delivery shapes a special kind of microbiome. So many studies describe that C-section babies have and we know the colonization is totally different. C-section babies have a delayed colonization of Bacteroidetes and lower diversity. So we can say vaginal delivers can provide enriched and diverse microbiome. In most European countries the rate of C-sections is higher than the 15% recommended by WHO. But we also know that C-section has a link to high risk to develop diseases like asthma, food allergies and obesity. And also, C-section are linked to antibiotic ex- position (mothers and neonates).
Maternal Diet
Nutrition had a major impact on early microbiota composition and function, with cessation of breastfeeding, rather than introduction of solid food, being required for maturation into an adult-like microbiota.
An intimate interrelationship between diet, the microbiome and immune system has been recognized to be associated with risk of Western diseases or NCD.
It is also described that maternal gut microbiota composition changes over the course of pregnancy. When transferred to germ-free mice, trimester 3 microbiota (T3) induced greater adiposity and insulin insensitivity compared to trimester 1 (T1) – the mice started to develop metabolic syndrome. (Koren et al., 2012 Cell Host)
An American study (Gohir et al. 2016 ) found that pregnancy-associated shifts in the ma- ternal microbiota are dependent upon the mother’s diet prior to and during pregnancy. A control diet and a high fat diet show a different profile and composition. Maybe we have now an intervention to modulate the microbiome of the mother transferred to the infants.
Pregnancy-associated shifts in the maternal microbiota are dependent upon the mother’s diet prior to and during pregnancy. Furthermore maternal diet is very important during lactation.
Breastfeeding
Many epidemiological studies demonstrate already that the breastfeeding practice protects infant against infections (Newburg,2009) and decreases the risk of developing respiratory tract infections, atopic dermatitis, asthma, obesity, type1 and 2 diabetes, NEC, gastroenteritis, and sudden infant death syndrome (Mayer-Davis, 2006; Amir et al., 2007; Kalliomäki et al., 2001).
Breast milk has an important impact on infant’s microbial colonization.
Milk contains different amounts of bioactive compounds including immunological compo- nents, proteins, peptides, hormones, bacteria, oligosaccharides, etc…. Milk hormones as leptin are related to energetic metabolism balance and they could help the prevention of overweight and obesity development (Aydin et al., 2008; Palou et al., 2009; Stoker and Cawthorne, 2008).
Human breast milk is a constant source of microbes which have an impact on infant’s microbial colonization. The infant is consuming around 104-105 cfu/mL shaping the micro- biome colonisation.
Recently studies suggest that Streptococcus and Staphylococcus genus are the predomi- nant genera in the human milk microbiota. These two genera may be universally pre- dominant and may have been underestimated in previous work using conventional PCR methods (Fitzstevens et al., 2016).
We know that microbes as fingerprint of the milk microbiome are highly variable between moms. Maybe we can think in some personalized food or personalize microbiome?
In collaboration with Seppo Salminen we analysed fecal samples from pregnancy and milk microbiome during the first 6 months in paired mother-infants dyads. We observed clusters in a total different manner. When colostrum milk microbiome changes to mature milk also these change is reflected in infant gut microbiome. (Unpublished)
A recent study investigated the association between breast milk bacterial communities and establishment and development of the infant gut microbiome (Pannaraj et al., 2017). It demonstrated that breastfed infants received 27.7% of their gut bacteria from breast milk and 10.4% from areolar skin during the first month of life. Changes in the infant gut micro- biome were associated with the proportion of breastfeeding in a dose dependent manner.
Perinatal factors affect breast milk microbiota composition
Milk microbiome is influenced by ...
sampling protocol; DNA extraction meth- od and sequencing platform Staphylococcus and Streptococcus spp. were equally abundant in both sample- type, Acinetobactersp. was higher in standard protocol, but not in strict aseptic protocol (Sakwinska et al., 2016).
Maternal weight and weight gain
There are higher diversity in normal weight milk samples and higher diversity in colostrum samples vs 1 month vs 6 month of lactation (Cabrera-Rubio et al., 2012). mode of delivery?
Human milk bacterial and glycosylation patterns differ by delivery mode (Hoashi et al. 2016). We had run a similar study in Spain and we also found differences between C-section and vaginal delivery (Ca- brera-Rubio et al., 2015 and 2012). However, other studies from Canada, China or USA did not report differences according to the mode of delivery (Urbaniak et al., 2016; Sakwinska et al., 2016; Pannaraj et al., 2017). Antibiotic use in C-section may play an important role in this reduction (Chart 2).
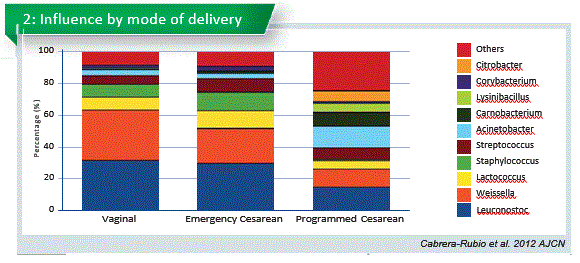
Emergency section is total different to the program C-section. Emergency section microbiome is similiar to the vaginal delivery. That opens new possibilities.
Geographical location difference?
We make a big study with milk samples from Spain, Finland, South Africa, China and we found cute differences in the microbiome. Milk microbiota differed in all the countries (p=0.002) suggesting a geographical area-dependent microbiota. We observed that some of the countries are more sensitive to delivery than to other. maternal genotype?
Comparing secretors and non-secretors genotype, we already know that oligosaccharide profile is totally different, we have observed that the microbiome profile was different. Not in composition but in quantity. Similar to that infants fed by non-secretor mothers have a delayed establishment in Bifidobacteria-dominated microbiota. other compounds in milk as macronutrients profile in breast milk?
But also we are really interested to identify if there are some relationship between in microbs and other compounds of the milk. In this study we check the relationship between microbes and macronutrients and we found association between fat and Staphylococcus and also bacterial load and Pseudomonas (Boix-Amoros et al., 2016). Recently studies also had been suggest connection between oligosaccharides profile and with a specific bacteria present in milk. Further investigation is needed.
Conclusion
Maternal microbiome, environmental factors and genetics have an impact to the infant’s microbiome
Through the lactation human milk as a life and complex food have an important role in the infant microbiota colonization.
Furthermore all the complex relationship between the biotic component present in milk are uncovered. And we don’t know the biological effect on the infants.
The role of bacteria in infant will help us to develop potential dietary strategies aimed at the beneficial microbiological, immunological and metabolic programming of child health.
Human milk oligosaccharides – unique composition and metabolism in infants
The speaker: Clemens Kunz, Germany
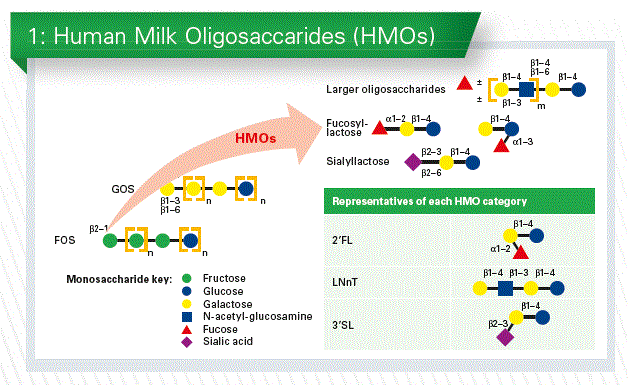
Besides lactose and fat, human milk oli-gosaccharides (HMOs) belong to the third most abundant group of milk components. Their monosaccharide composition and linkages are rather unique. HMOs are currently the most important nutritional gap between human milk and infant formula.
Today, we are at the beginning of a new era as the large scale production of some HMOs is possible. HMOs are one of the hottest topics in infant nutrition. This research area is rather complex due to the structural diversity of HMOs. There is already a very high number of publications based on in vitro or animal studies. Although these data are exciting, they still have to be verified for infants. The fascinating point is that the first infant studies investigating an infant formula with one or two individual components added have just been published. These two components are identical to those occurring naturally in human milk.
Fundamental questions are:
What is so unique about HMOs? Are there similarities between HMOs and current prebiotics in infant formula? What are the special functions of HMOs (local and systemic effects)?
Is the HMO pattern and secretor/ non-secretor status linked to disease prevention?
What is the advantage of supplementing infant formula with HMOs?
The total amount of HMOs ranges from about 5 to 15 g/L. The quantity of these components does not only depend on the lactational stage of the mother but is also affected by the expression of specific glycosyltransferases in the mammary gland, which makes human milk a very unique biological fluid. In contrast to animals in humans genes encoding for blood group H and Lewis antigens as well as the secretor status determine the presence of a1-2-, 1-3- and/or 1-4-fucosylated oligosaccharides.
If we analyze milk from various women, the milk composition will differ. The question is whether these differences have an impact on outcomes and health. Apart from that it is usually not only important the total amount of HMOs an infant receives but also the kind of HMO pattern?
In many publications on prebiotic oligosaccharides (PBOs), these components are compared with HMOs. However, from the structural point of view PBOs and HMOs do not have any similarity at all. Like many others (e.g. fiber, dextrin or glucose), both belong to the group of carbohydrates, however, their biological functions are not categorically the same.
What is so unique in the HMOs structures?
A breastfed infant receives HMOs which are of extremely high structural diversity with more than 150 components. We will not be able to imitate this complexity, but the unique structures of each individual component are interesting enough to discuss specific functions. Very simply, HMOs consist of five monosaccharides only: glucose, galactose, N-acetylglucosamine, N-acetyl-neuraminic acid and fucose. Almost all of them consist of lactose as backbone. What makes them so unusual is their specific linkage with each other, which predisposes them to various functions.
What are their functions?
If an infant receives HMOs, these will most likely influence the microbial composition in the colon, a topic which is heavily investigated today (Chart 1). They also can interact with the attachment of bacteria to the cell surface in stomach, intestine and colon, an effect which depends on the bacterial preference to recognize specific carbohydrate ligands. Or they might influence gut maturation. Under certain conditions they even may change the surface glycosylation and hereby influence the defense system.
Research in the bifidogenic effect of HMOs is of great interest to many. Fecal samples of breastfed infants usually, but not always, contain a high number of Bifidobacteria and a low number of other potentially pathogenic bacteria. In fecal samples of formula-fed infants the microbial composition is often different. Besides the interaction of HMOs with Bifidobacteria, the prevention of pathogen adhesion to the epithelial cells by HMO via blocking the adhesion sites are even more specific and very important for the infants´ defense system.
There are also discussions that HMOs may have systemic effects, influencing inflammatory or immune processes or brain activity, e.g. by influencing ganglioside composition. However, the available data on brain composition are not convincing yet.
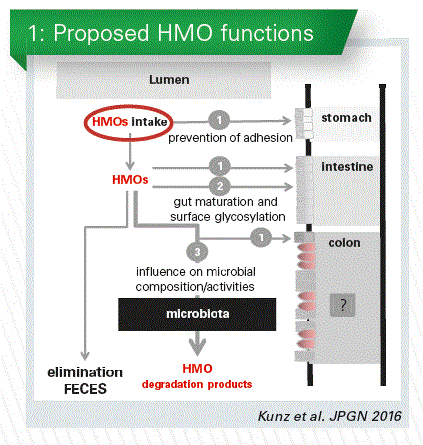
What about intake and metabolism?
In many publications it is stated that HMOs resist digestion, reach the colon intact and then are available for the microbiota. But is this true for all HMOs in human milk? Within this context it is hypothesized that a decrease in fecal HMOs indicates a preferred utilization by the infant microbiota. Therefore, it is important to know which amount and what kind of HMOs an infant receives? We need to know what is used by the microbiota and what is excreted in the feces and urine. It is obvious from metabolic studies that some HMOs are absorbed and, hence, might have systemic effects.
Studies with infants are difficult to do. Some years ago we raised the question “How can we get more information on the metabolic fate in infants?”. We finally applied stable 13C-galactose to lactating mothers for individual labeling of HMOs. We could show that this galactose was indeed transported into the mammary gland and not only taken up by the liver. And then we could show that this label is incorporated into lactose and oligosaccharides. Thus, we were able to do metabolic studies, analyzing not only milk but urine and fecal samples as well. We have then been able to relate those to each other. We collected milk, urine and feces samples from many mothers and infants at each suckling over 36 hours. For the first time we have thus been able to quantify the intake and excretion to get real quantified data.
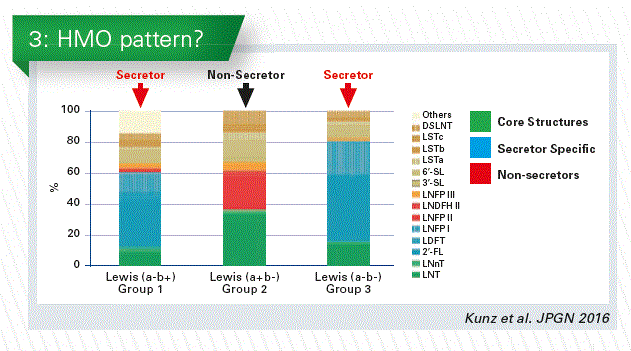
In a recent cooperation with Maria Carmen Collado and Cecilia Martínez-Costa we have done a study on the differences between term and preterm infants. There seems to be no difference in the total HMO content, and no difference in neutral and acidic HMOs as well. However, if we redistribute those samples according to the secretor and non-secretor status of the mother, we will find a large difference in the total amount (Chart 2). This is caused by the differences in the neutral fraction.
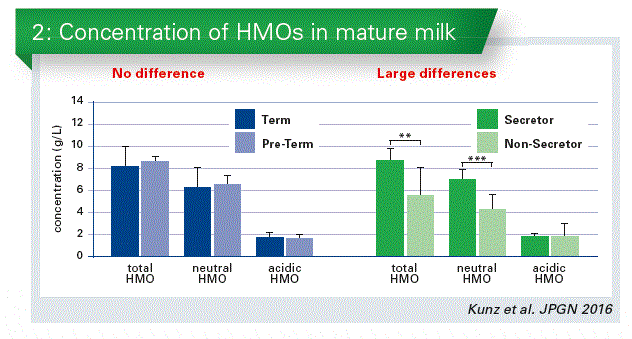
Secretor/Non-secretors specific status
This secretor/non-secretor status is very important, because it is discussed to have an influence on certain diseases, e.g. on the development of Crohn’s Disease or Norovirus infections. We all belong either to secretors or non-secretors, irrespective of whether we are lactating or not. In Europe e.g. about 80 % of the population belong to so-called secretors; 20 % are non-secretors. Secretors express a1-2-fucosylated carbohydrate epitopes on their cell surfaces, e. g. on intestinal cells, on erythrocytes etc. Those epitopes are also secreted into biological fluids such as milk. Non-secretors do not have these specific carbohydrate linkages.
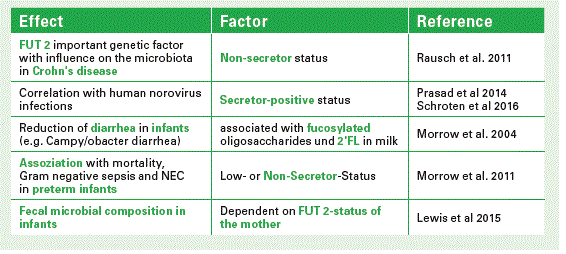
In secretor milk 2’FL is present in large amounts. Together with the core structure LNT and two other a 2-fucosylated structures these components comprise between 60–80% of all HMOs (see Chart). If you compare the secretor pattern with that of non-secretor milk it will be completely different. And it is not only the pattern which is different, but the total amount also varies considerably (Chart 3).
I already mentioned that an infant receives about 5-15 gram HMOs per liter milk. Most of it, I would say, is used by the microbiota, but we and others found a fecal excretion of intact HMOs or degraded components. Some HMOs are also absorbed as we identified these structures in infants´ urine. For example, we quantified single HMOs such as LNT and LNFP II and observed that after each suckling 50–150 mg of a single component is excreted in urine. That means HMOs circulate in the blood being available for cells if they need them for specific functions.
Conclusion
HMOs are very complex and unique components, not to compare with other prebiotics on the market.
Structural diversity of each single HMO predisposes them to specific, multifunctional effects.
Few HMOs (n=5–7) comprise the majority of all structures.
Data from studies of single HMO components should carefully be investigated and studies need to be reproduced.
HMO – results from clinical research in infant nutrition
The Speaker: Philippe Alliet, Belgium
HMO are the third most important component in human milk, more important as solid than protein fraction. And that is a big difference with infant formula and cow‘s milk, in which this part is not present. The oligosaccharides are very complex, very abundant and the proportion is not similar during the whole period of lactation.
More than 150 different HMOs have been identified. To more simplify the 150 we can according to the different monosaccharides present them in three categories (Chart 1).
Two of those are more abundant: 2`FL and LNnT. And from in vitro studies we know that there is accumulating evidence suggesting that they may support gastrointestinal and immune functions of breastfed infants. The mechanisms that have been put forward are the effect of the microbiota growth, function and establishment, protection from infection and effects on allergy and immune competence. One of the ways why people think that they might help against infections and bacterial infections is that they have similarities with some receptors of certain gastrointestinal bacteria and they could interact or prevent the adherence of those microbes to the receptors in our gut.
We performed in two centers in Palermo, Italy, and in Hasselt, Belgium, this randomized controlled trial with infant formula supplemented with 2 synthetic human milk oli- gosaccharides (2’fucosyllactose [2’FL] and Lacto-N-neotetraose [LNnT]). The overall objective of that safety study was the effect of this formula supplemented with these 2 synthetic human milk oligosaccharides on growth, digestive tolerance and morbidity in healthy infants. For the microbiota related objectives we compare microbiota profile and metabolic signature among the infants given the test formula (EF) and the control formula (CF) and there was also a non-randomized exclusively breastfed infants group (BF).
The hypothesis was that microbiota profile and metabolic signature in the infants fed EF (vs. CF) will be closer to that of BF infants.
Infants were enrolled before the age of 2 weeks. After informed consent they got a medical examination and anthropometry. Then they were randomized towards the test or the control formula (Chart 2).
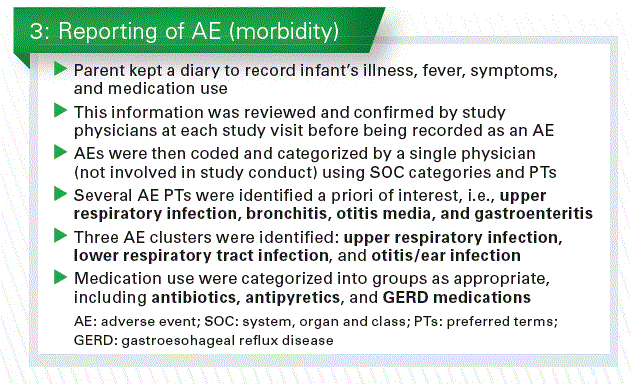
They were seen at 1, 2, 3, 4 and 6 months, which was the end of the treatment. All infants received a standard follow-up formula from 6-12 months and were seen again at 12 months. Complimentary food was only started at 4 months of age, digestive tolerance and adverse effect were recorded in a parent’s diary (Chart 3).
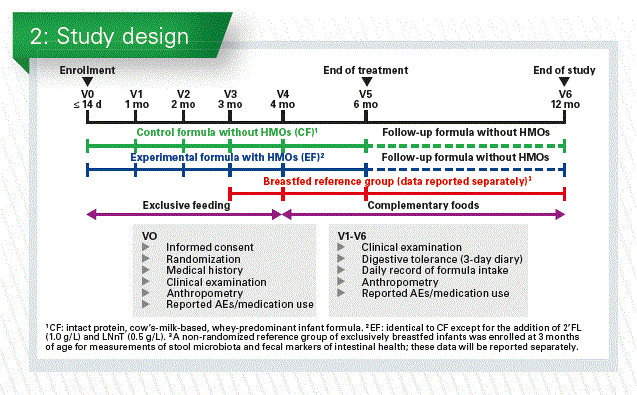
Baseline characteristics
175 infants were included, most of them were followed up at 6 and 12 months. Baseline characteristics were comparable between EF and CF groups. Mean difference in weight gain (EF vs. CF) was -0.30 g/day (95% CI -1.94–1.34, p=0.72), within the non- inferiority margin. Infants receiving EF (vs. CF) did not differ in weight, length, head circumference, BMI, or corresponding z-scores through 12 months.
Digestive Tolerance
Measures of digestive tolerance were generally similar between groups.
- no difference in stool consistency, except at 2 mo (softer in EF vs. CF)
- no difference in stool frequency between EF and CF groups
- GI symptoms and behavioral patterns were similar between groups
The bacterial composition
There was a big distinction between EF and CF groups.
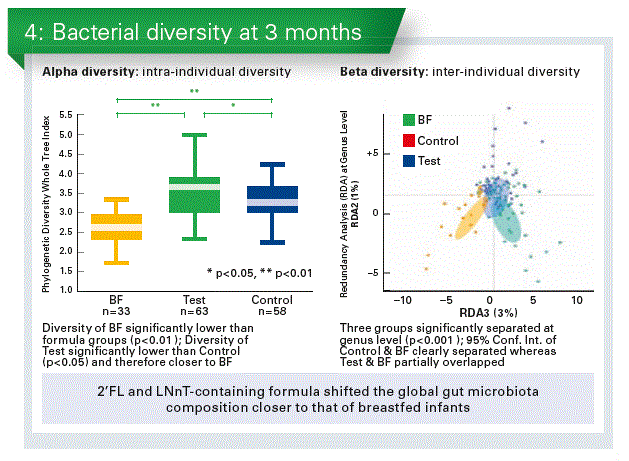
- 2’FL and LNnT-containing formula shifted the global gut microbiota composition closer to that of breastfed infants (Chart 4).
- Formula with 2’FL and LNnT promoted the colonisation of potentially beneficial Bifidobacterium and reduced taxa with potentially pathogenic members.
Stool metabolic signature of EF was closer to BF, consistent with the findings in bacterial composition and diversity. - Altered bacterial composition may result in reduced protein fermentation in EF vs. CF.
Clinical results
The only way we could look at the clinical results in this study was by using the reported adverse events, identified a priori, and medication use.
- Infants receiving EF (vs. CF) were less likely to experience bronchitis through 4 months (OR 0.16, 95% CI 0.02–0.78, p=0.010), 6 months (p=0.005) and 12 months (p=0.004), and lower respiratory tract infection through 12 months (OR 0.45, 95% CI 0.21–0.95, p=0.027).
- Infants receiving EF were also less likely to receive antipyretics through 4 months (OR 0.44, 95% CI 0.20-0.98, p=0.032), and antibiotics through 6 months (OR 0.53, 95% CI 0.27-1.02, p=0.047) and 12 months (p=0.016).
Of course we have to be careful with the interpretation of these results since they were secondary endpoints of the study.
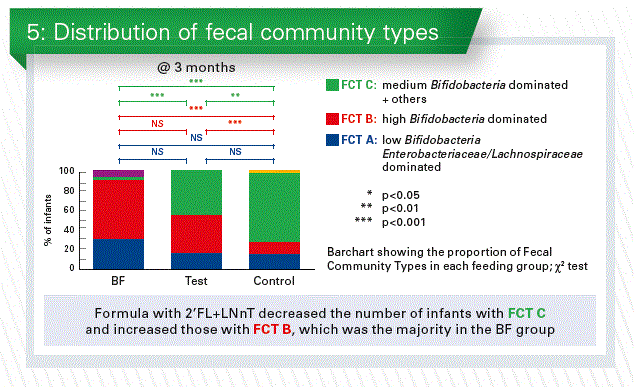
Fecal community types in infants at 3 months
If you put all data of the enrolled infants together you can find 3 types of fecal community type (FCT), namely
- FCT A: low bifidobacteria, high enterobacteriaceae/lachnospiraceae
- FCT B: high bifidobacteria and low others
- FCT C: medium bifidobacteria and also the others
When we now looked in the three groups in our study (EF,CF, BF) we see again a big difference between the breastfed and formula fed. But it seems that in the test group (EF) the FCT B was higher and the FCT C was lower than in the control group (CF (Chart 5).
And if we compare that to the antibiotic use it looked that especially the FCT C was more prominent in the group that was receiving the antibiotics. All those data seem to go into the same direction and are concordant to the in vitro data.
But this of course need further research in order to confirm these results.
Conclusion
First clinical data demonstrate that synthetic HMOs are safe and well tolerated (structurally identical to those found in human milk)
First clinical data show that formula with 2’FL + LNnT promotes the Fecal Community Type B seen in breastfed infants.
The reported reduced likelihood of antibiotic use with 2’FL + LNnT may be linked to the Fecal Community Types
After these first promising health outcomes, further studies are needed to establish efficacy
Part II: Nutrition and Healthy Growth
Human milk – the best infant nutrition and health protection
The speaker: Massimo Agosti, Italy
The first 1000 days are a very dynamic period with a lot of changes. And the real important protagonist that covers this period is human milk or better breastfeeding. Because human milk is a component but breastfeeding is an action.
Exclusive breastfeeding for the first 6 months of age, with continuation for 1 to 2 years of life or longer, is recognized as the normative standard for infant feeding. The composition of human milk is the biologic norm for infant nutrition. Human milk contains many hundreds to thousands of distinct bioactive molecules that protect against infection and inflammation and contribute to immune maturation, organ development, and healthy microbial colonization.
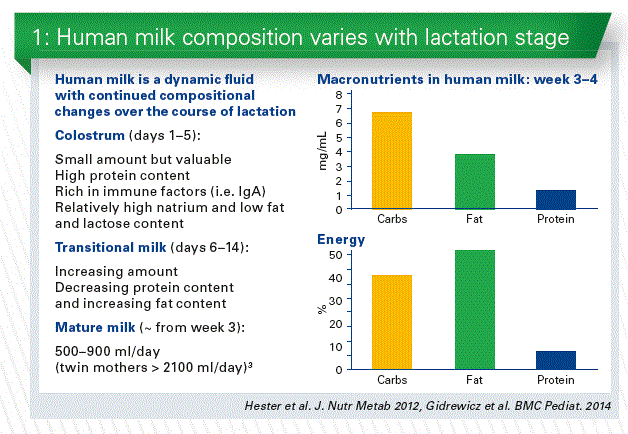
Mother milk composition is dynamic, and varies over the lactation, influenced by many factors. Average milk volume varies between mothers. Milk from different mothers is never identical in all components, not even in the same lactation period. Even the nutrient content of different milk samples taken from the same mother is not identical (Chart 1).
Unlike infant formula, which is standardized within a very narrow range of composition, human milk composition is dynamic, and varies within a feeding, diurnally, over lactation, and between mothers and populations. Many studies of human milk composition have been conducted in different populations using varied collection, storage and testing methods. Influences on compositional differences of human milk include maternal, environmental factors as well as many other factors.
Geographical Origins
For human milk we see different composition in total amino acids content and in free amino acids content in different countries. For free amino acids we have e.g. more glutamat in the milk of western breastfeeding mothers, especially in the USA. Probably dietary factors could play a role. We cannot disclude the diets how much content could influence about that.
Gestational Age
The content in proteins, especially in the first weeks depend on weeks on gestation about:
Protein levels decrease in human milk over the first 4 to 6 weeks or more of life regardless of timing of delivery. Human milk protein concentration is not affected by maternal diet, but increases with maternal body weight for height, and decreases in mothers producing higher amounts of milk. In the same way amino acids differ in the milk of term and of preterm babies.
DHA is an essential building block of brain and retina and plays a large part in the development of the brain and visual function in babies, especially for preterm. The DHA content of breast milk is strongly influenced by the mother‘s diet.
We also know the difference in the gestational age for some micro- nutrients. See for instance the difference for vitamin A and E in comparison term with preterm babies.
Gender diversity
The gender of the baby plays a role. Mothers of male offspring produce milk with a higher energy content.
The average energy content of breast milk formale offspring is higher than that of milk for female offspring by around 25 kcal/100ml during pregnancy. For an average milk intake of 800 ml/day this means 200 kcal/day.
Mode of delivery
Also the mode of delivery plays a role. The different content is higher in vaginal than in cesarean delivery.
Protein content
About the protein content we know that the total concentration in amount is different and decreases throughout lactation. Protein requirements for growth decreases with the slowing growth rate. The protein content decreases throughout lactation, answering in some way to the different protein requirements.
Bioactive protein
Human milk contains partially hydrolysed proteins. To date, around 200 different peptides have been identified in breast milk.
“In other words, the mother provides the infant with not only dietary proteins but also the means to digest them.” (Dallas et al., 2015)
Human milk provides protection against infections and helps the infant’s immature immune system develop. Colostrum and early milk is rich in immunologic components such as secretory IgA and lactoferrin as well as developmental factors such as epidermal growth factor. Primary functions of colostrum are immunologic and trophic rather than nutritional (Chart 2).
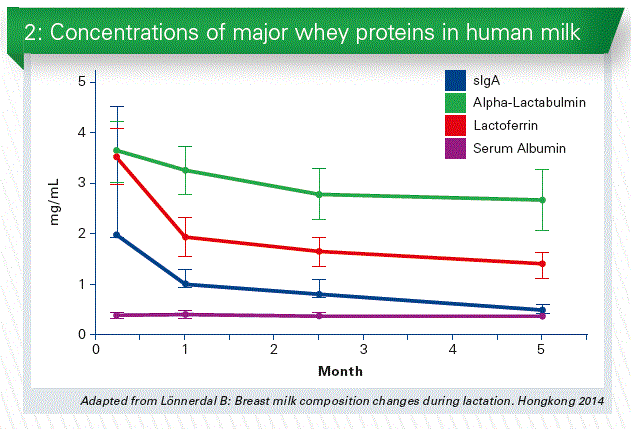
Carbohydrates
The concentration of lactose in human milk is the least variable of the macronutrients, but higher concentrations of lactose are found in the milk of mothers producing higher quantities of milk (Human Milk Composition: Nutrients and Bioactive Factors, Ballard et al., 2013)
Human milk contains a variety of cells, including macrophages, T cells, stem cells, and Lymphocytes. Vaginal delivery is associated with higher colostrum protein content. The principal sugar of human milk is the disaccharide lactose. The other significant carbohydrates of human milk are the oligosaccharides, we heard a lot on this morning.
Breast milk immune factor composition varies, not only depending on the length of lactation, but also on gestational age. Maternal lactogenic compensatory mechanisms only accelerate the development of immature breastfed preterm infants after 30 weeks of gestation, not earlier.
Arachidonic acid (ARA) and docosahexanoic acid (DHA) are of major importance for neural maturation and retinal function in infancy. Requirements in preterm infants are increased due to accelerated growth and limited body stores.
Skin-to-skin contact
Human milk is not merely a nutrition act.
Breast milk contains not only a variety of physiological but also psychological factors with qualities that have a profound role in infant survival and health. We know the action of breastfeeding is a relationship between mother and baby. In fact a mother, giving her breast milk, not only she is feeding her baby, but is also creating a relationship model which is internalized between mother and baby, which will serve as a basis for all subsequent intimate and social relationships of the individual. With breastfeeding the baby experiences affectivity, warmth, contact, love and a very intense mother’s care.
We know the bonding theory on the importance of skin-to-skin contact. We cannot divide contact from feeding milk. It is considered essential for mental health that infants experience a warm, intimate, uninterrupted relationship with their mother in whom both can find satisfaction and enjoyment“ (J. Bowlby, 1988)
Utilizing this contact of prolonged bonding we can create more contact between mother and infant. As well is more important more sense of effectiveness and security as mother, best adaptation to maternal role and so on. In 2016 the American Academy of Pediatrics recommends the immediate skin-to-skin contact for newborn.
From a practical point of view we can say that for instance if you analyse preterm baby that had skin-to-skin contact or not you can see different microbiome in the mouth oft he baby.
There are some other studies and it is interesting we could see a difference.
Probably we also have to account the effect of skin-to-skin contact for the mother. In an Australian retrospective study with 7.548 patients we can see that women who did not skin-to-skin contact or breastfeeding had a double risk of postpartum hemorrhage. (Saxton et al., 2015)
Conclusion
In breastfed infants:
Mother’s milk composition is dynamic, varying during the lactation time, during the same day and the same feeding, and it is influenced by many factors.
Some of these changes in nutrient concentrations reflects the successively slowing growth rate and developmental changes in metabolic requirements that infants undergo during the first year of life.
Differences still persist between breast feeding and formula feeding and their outcomes. To reduce that we need a continue increasing knowledge of infant nutrition.
Protein sources in infant formulas. What is the standard?
The speaker: Yvan Vandenplas, Belgium
Partially hydrolysed formulas for every healthy infant? That is a quite provocative question. Breastfeeding is an unequalled way of providing ideal food for the healthy growth and devel- opment of infants. But we also know that not every infant can be breastfed and I think that every non-breastfed infant has also the right as optimal second choice.
Traditionally we use cow’s milk based infant formula for the simple reason that there are many cows which provide a lot of milk. Not because cow’s milk resembles mother milk. There are huge differences.
So cow’s milk needs to be adapted to the needs of infants to become a valuable second choice infant feeding. We have to change also the structure of the proteins by hydrolysing. We have been using partially hydrolysed formulas (pHF) mainly in the prevention of allergic dis- eases and they are also used in therapeutic formulas for functional gastrointestinal disorders. And the question is, should we use pHF for every infant as the second best option after breastfeeding?
It is to consider:
- Partial hydrolysates are “specific”
- Results from one hydrolysate cannot be extrapolated to another
- If all studies are grouped together, with poor quality and different hydrolysates: no preventive effect
- If studies are selected on hydrolysate of high quality studies with one specific hydrolysate: preventive effect on atopic dermatitis (AD) and probably also cow’s milk allergy.
Association of colic and GI symptoms
There are studies with pHF and functional gastrointestinal disorders (FGID). And if allergy is considered 3–5 % of the population of infants, gastrointestinal disorders are of course much more frequent: 50 % of all infants worldwide will develop at least once FGID. And they are all interrelated to each other and so for the primary health care physician it is often very difficult to know what is the starting gastro intestinal symptom.
Looking at data where you separate infants with colic and those without, you will see that the other GI symptoms are all at least two times more frequently present, than in infants without colics. (Vandenplas Y, 2017) So the question is: Are pHF also useful in the treatment of FGID?
Regurgitation
We looked at the frequency of regurgitation according to literature. There are 13 studies and we found a medium value of 26 % of all infants which develop trouble with regurgitation. The mean of the suggested estimation of 500 different pediatricians worldwide, asking the same simple question: How frequently do you think it occurs? The answer is quite close to the medium value of the literature.
Do we have to treat infant regurgitation, are there negative longterm outcomes? The data is very limited, there is only one study (Martin AJ, 2002). If you are spitting 3 months during your first 2 years than you have a significant high risk to have reflux symptoms at the age of 9 years (Chart 1).
Whether an intervention during early life has an impact or not, we simply do not know. There is only one study in literature which com- pared thickened formula with intact protein with thickened formula with partial hydrolysed whey formula (Vandenplas Y, 2013). Statistically the pHF may do better.
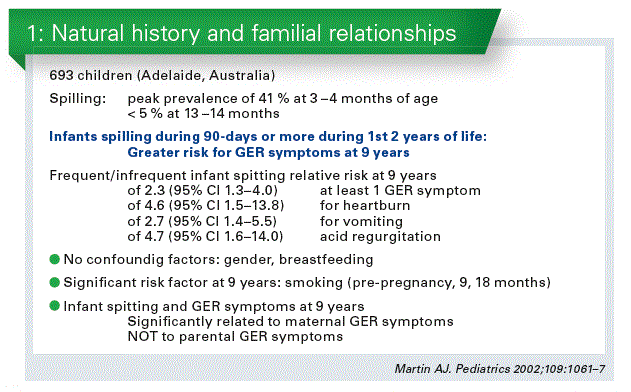
Constipation
Same data from that analyses from literature how frequently it does occur. About 10% of the babies develop functional constipation as medium value. There is only one study looking at the efficacy of pHF as single intervention in the treatment of infant constipation which looked at healthy infants with a number of frequency of defecations. There were two times more defecation/day with a pHF, that brings the number of defecation nearer to the number of defecations of breastfed infants. (Hyams at al., 1995) (Vandenplas Y, 2015).
Infantile colic
20% of all infants develop infantile colic according to literature. A recent study data comes exactly to the same conclusion. There is no study in prevention of colic with pHF and there is also no study with only change of intact protein with pHF. There are several studies on efficacy but with other changes of formula composition that are indicating there might be some benefits.
Can we recommend pHF for every healthy baby?
What most societies actually recommend is to use pHF in at risk babies, and we define “at risk babies” as an infant born in a family with a history of atopic disease with at least one first degree relative with atopic disease (AD). But these are data from a study of Northern France with 200 babies responding to this criterium. And just half of the babies did not get the pHF or were breastfed. So we do not follow the guidelines, because simply the family history is not taken soon after birth. That is one weakness of the recommendations. A second recommendation is, to give pHF in those infants with high and medium risk. In fact, most of the studies are performed in families with high risk of AD. 5% of the babies are born in this situation. They have a genetic risk at 50–80% to develop AD. We extrapolate that to also include infants born in a family with a medium risk. That are 20-40% of all the babies developing AD. However, if you look at babies born in family with a low risk – that are about 70 %. We recommend today pHF only for the half of the children who develop AD (Chart 2).
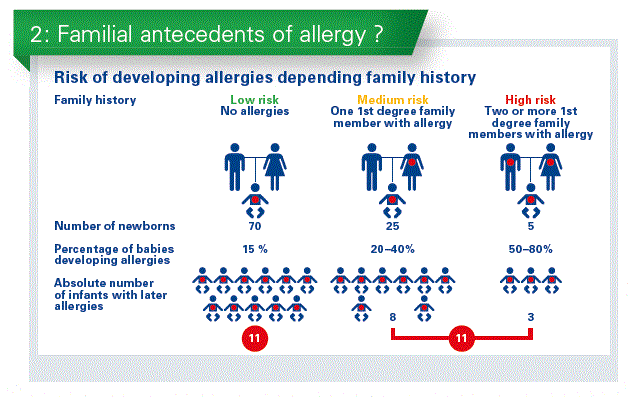
If pHF is safe and there are no adverse events why not use it for every baby?
There was only one study performed in two Swiss cities with non at risk situation to compare pHF to intact protein. So there was in one city, where there was an intervention, in the other one no intervention. The 2 groups are more than 500 infants in each city. The results may scientifically relatively weak, but the outcome is the same for all infants. In the group without intervention, health problems increases from 33% to 49% compared to 27% to 32% between 3. and 6. month. That is the only study we have on non at risk infants.
Some studies have shown, that economically for the Ministry of Health, insurance companies and the families pHF is cheaper because it is associated with a decreased incidence of diseases (Spieldenner J, 2011/Su J, 2012).
A side study from the GINI study shows that also infants, who have negative family history of AD, when they were born. These infants have the same risk when a first degree relative develop AD later. It is exactly the same when that occurs before the birth of the child.
So what we use as family history is in fact poor selection criteria. It is the genetic background which is important, and not if you have developed symptoms or not at the moment when the child is born.
Moreover there is no intact cow’s milk protein in breast milk. And is there even “no intact protein” in breast milk, as breast milk also contains proteases to hydrolyse “immediately” the protein in breast milk. We do not know the size of the proteins. So do we really need to give intact protein to the baby? I my opinion, today that is a individual decision of the pediatrician.
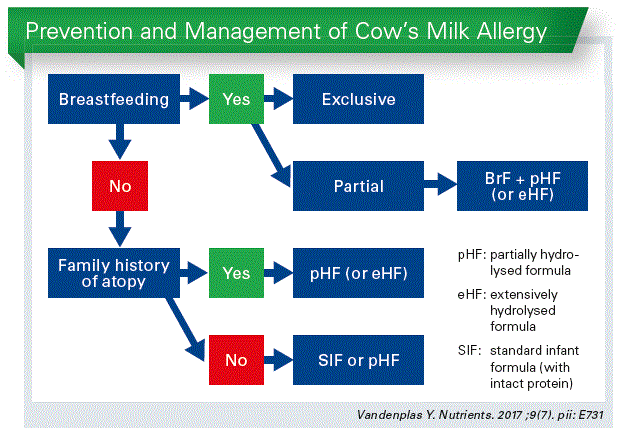
Conclusion
Partial hydrolysates are clearly not the same, they are “specific”
Partially whey HF may offer a useful alternative to intact protein in the dietary management of common functional gastrointestinal symptoms (Consensus paper – Vandenplas Y et al. 2014)
Based on available data, the use of pHF in healthy infants is safe with regard to growth. (Consensus paper – Vandenplas Y et al. 2016)
Most societies have accepted pHF as a safe protein source for infant formula for every infant.
There are a lot of studies that show there were benefit or no benefit. But there is not one study which shows worse results for pHF and all studies show that is very safe.
The role of meta-analysis in the evaluation of the effects of hydrolyzed formula for allergy prevention
The speaker: Hania Szajewska, Poland
Hierarchy of evidence
In the hierarchy of levels of evidence, the results of a systematic review, with or without a meta-analysis, are considered to be the evidence of the highest grade. Thus, if available, systematic reviews and meta-analyses should be used in support of clinical decision making (Chart 1).
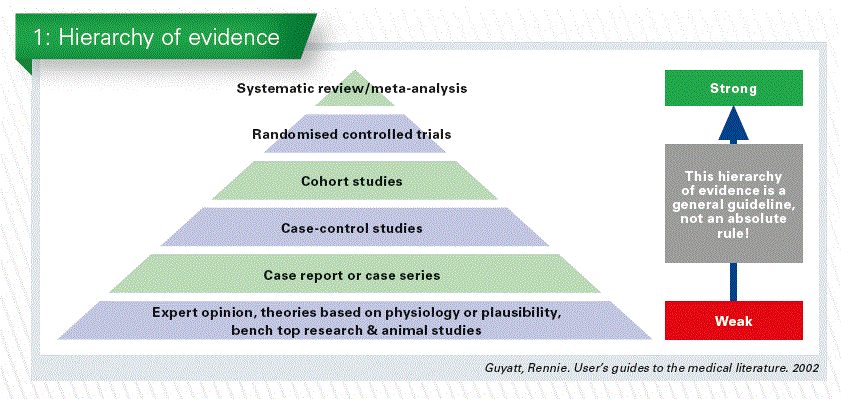
Systematic review or meta-analysis?
While the two terms, i.e., ‘systematic re- view’ and ‘meta-analysis’, are commonly used interchangeably, there is a distinction between them. A systematic review is a review of a clearly formulated question that uses systematic and explicit methods to identify, select and critically appraise relevant research, and to collect and analyze data from studies that are included in the review. Statistical methods may or may not be used to analyze and summarize the results of the included trials. A meta-analysis is a name that is given to any review article when statistical techniques are used in a systematic review to combine the results of included trials to produce a single estimate of the effect of a particular intervention.
Why perform a meta-analysis?
The major reasons to perform a meta-analysis are as follows:
- to increase power (i.e., the chance to reliably detect a clinically important difference if one actually exists). The problem with many individual studies is that they are too small to detect small effects, which can only be detected if the data from several trials are combined;
- to improve precision in estimating effects (i.e., to narrow the confidence intervals around effects);
When to pool the results?
One of the most important and controversial decisions to be made by reviewers is to decide whether to pool the results of individual trials. The take-home message is that it is always appropriate to perform a systematic review, and every meta-analysis should be preceded by a systematic review. However, not every systematic review should be finalized with a meta-analysis; in fact, it is sometimes erroneous and even misleading to perform a meta-analysis. In principle, data only be pooled if the data summarized are homo-geneous [i.e., the participants, intervention, comparison, and outcomes must be similar (homogeneous) or at least comparable].
Hydrolyzed formulas
There are two major types of hydrolyzed formulas. These are partially hydrolyzed formulas (pHF) and extensively hydrolyzed formulas (eHF). Current classification of infant formula focuses on the degree of hydrolysis; however, different manufacturers employ a number of different proprietary methods of hydrolysis. In general, pHFs have peptides which are less than 5 kDa with a size distribution of 3 to 10 kDa, while eHFs have peptides which are < 3 kDa. Of note, there is no agreement on the criteria on which to base this classification because the process of degree of hydrolysis is in fact a continuum between the intact protein at one end and amino acids at the other end.
The role of hydrolyzed formulas for allergy prevention
Most guidelines and experts recommend that infants with a documented hereditary risk of allergy (i.e., an affected parent and/or sibling) who cannot be breastfed exclusively should receive a formula with confirmed reduced allergenicity, i.e., a partially or extensively hydrolyzed formula, as a means of preventing allergic reactions, primarily atopic dermatitis. However, a 2016 meta-analysis by Boyle et al.* questioned the role of hydrolyzed formula for the prevention of allergic disease(Chart 2). This meta-analysis found no consistent evidence that use of pHF or eHF reduces the risk of allergic outcomes in infants at high pre-existing risk of these outcomes.
While the authors of this meta-analysis evaluated separately partially and extensively formulas, various types of hydrolyzed formulas in each category were combined. However, not all hydrolyzed formulas are equal. The review included not only RCTs but also observational studies. As the reviewers subjectively chose the time intervals of assessment (0–4, 5–14, and ³ 15 years), a number of decisions were made as to which data should be used for their analyses. Furthermore, the authors included studies in which in the intervention group, but not in the control group, additional interventions were applied such as house dust mite control measures and a smoke-free environment.
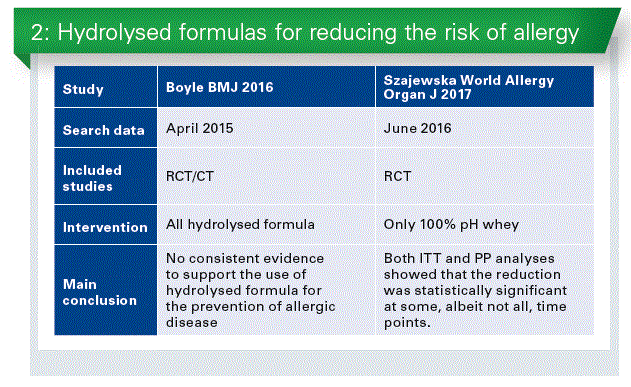
Different approach
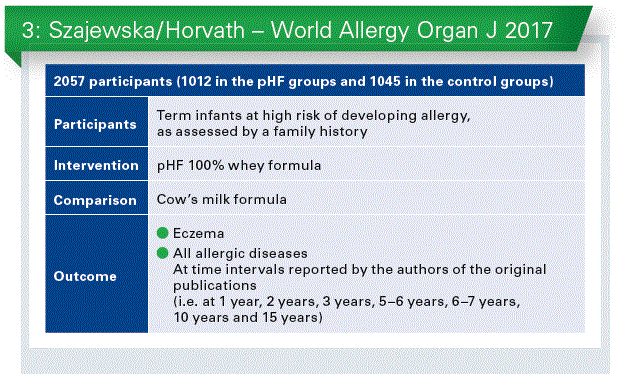
As not all hydrolyzed formulas are equal, the efficacy of each hydrolyzed formula should be established separately. This is because factors such as the protein source, hydrolysis method, and degree of hydrolysis that often depend on the manufacturer contribute to differences among hydrolysates. Recently, we up-dated evidence on the effectiveness of using 100% whey pHF, manufactured by a single manufacturer, for reducing the risk of eczema and allergy in healthy infants at high risk for allergy.** Thirteen publications reporting on 8 RCTs were included (Chart 3). Use of pHF compared to cow’s milk formula reduced the risk of eczema and all allergic diseases among children at high risk for allergy. Both intention-to-treat (ITT) and per-protocol (PP) analyses showed that the reduction was statistically significant at some, albeit not all, time points. There is evidence to consider use of pHF as an option for reducing the risk of any allergic diseases, particularly eczema. However, the certainty of the evidence is low.
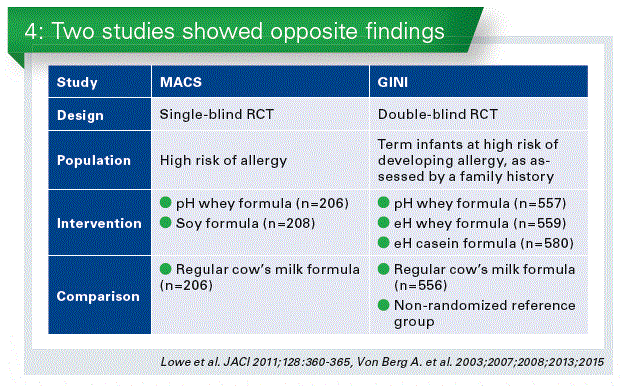
One characteristic that makes this meta-analysis distinct from other reviews is that it focuses exclusively on only one type of pHF. The results of both the ITT (more precisely, available case analysis) and the per-protocol analyses, as they complement each other were presented. In the 2 largest studies (i.e., the GINI and the MACS), the rate of breastfeeding was high (approximately 40%) (Chart 4). Thus, the ITT analyses included infants who were exclusively breastfed, including infants who were never exposed to pHF. The per-protocol analysis included all participants who adhered adequately to the assigned regimen. While not ideal from a methodological point of view, the per-protocol analysis is important for understanding the role of pHF in allergy prevention, and hence, the decision to include both.
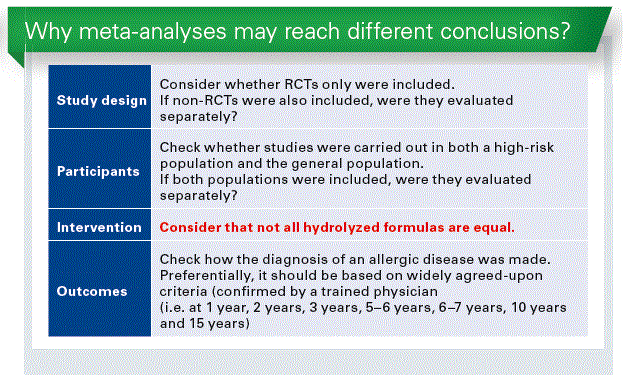
Conclusion
There are specific considerations when interpreting findings of a meta-analysis evaluating the role of hydrolyzed formulas.
Not all hydrolyzed formula are equal.
The efficacy of each hydrolyzed formula should be evaluated separately.
References:
* Boyle RJ, Ierodiakonou D, Khan T, Chivinge J, Robinson Z, Geoghegan N, Jarrold K, Afxentiou T, Reeves T, Cunha S, Trivella M, Garcia-Larsen V, Leon- ardi-Bee J: Hydrolysed formula and risk of allergic or autoimmune disease: systematic review and meta-analysis. BMJ. 2016 Mar 8;352:i974
** Szajewska H, Horvath A: A partially hydrolyzed 100% whey formula and the risk of eczema and any allergy: an updated meta-analysis. World Allergy Organ J. 2017 Jul 26;10(1):27
Part III: Preterm Nutrition
Role of Iron in Preterm Infant Nutrition
The speaker: Walter A. Mihatsch, Germany
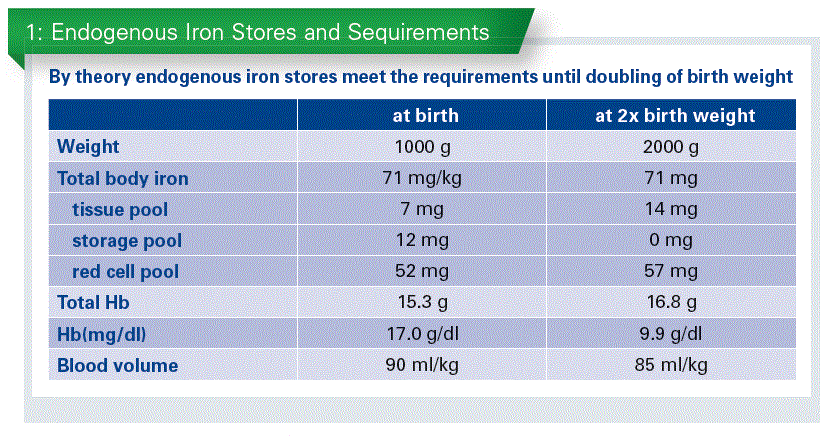
The iron stores in preterm infants have been analyzed in the 1950s by Ellie Widdowson. Her data suggested a more or less linear association between body weight and iron content. (Widdowson EM et al., 1951).
That is a very important point. Theoretically as soon as the weight of a preterm doubles in our ward the total body iron content should double as well. Ellie Widdowson’s data suggested that the total body iron at birth is between 65–75 mg/kg. So a 24-weeker of 700 g will have 52 mg iron, and an infant near term with 3,100 g will have a total body iron of 232 mg. There are certain situations where iron stores are reduced e.g. in a severe maternal iron deficiency, maternal diabetes mellitus (Petry J, 1992), IUGR (Georgieff J, 1995) or chronic fetal blood loss (e.g. twin-twin-transfusion-syndrom). The final important mark is, that iron status at birth does not influence post-natal growth velocity (Sichieri R, 2006).
Iron is essential for preterm infants. They are at high risk of iron deficiency (ID) which may have adverse effects on brain development and function. Up to 75% of LBW infants have been reported to develop ID anemia within the first 6 months of life (Lundström U, 1977). Risk factors are prematurity and small size.
In his hallmark 1977 paper Lundström studied 171 infants with a birthweight of 1,000– 2,000 g. Half of them were supplemented with 2 mg/kg/d ferrous sulfate from 0.5–6 months. Adding iron improved haemoglobin and MCV.
When to start iron supplementation?
In absence of iron supplementation, by theory, endogenous iron stores meet the requirements until doubling of birth weight. At that point of time the iron stores will be exhausted (Chart 1).
The time to double birth weight depends on the age of gestation. An infant of 24 wks of gest. and around 700 g will need 6 wks. A term baby with 3,100 g will need 24 wks on average to double the birth weight. So, the smaller infants are, the more early they require iron for growth. This data has been reconfirmed by a recent study (Akkermans MD et al.,2016).
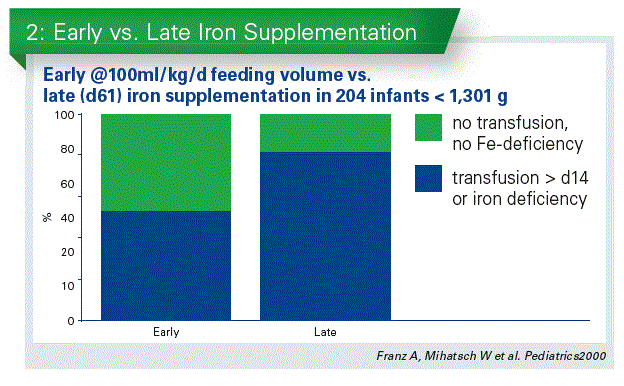
One of the most important studies of iron supplementation in marginally LBW infants randomized 285 preterm infants (2–2,5 kg) in three groups: placebo, 1 mg/kg/d iron and 2 mg/kg/d iron (Berglund S et al, 2010). With increasing iron intake, the ferritin increased as well and it was highest in the group, receiving 2 mg/kg/d iron. The important point of the study was, that with 1 mg/ kg/d iron, iron deficiency and anemia was observed in 7 % of the infants – this dose is not enough. It is difficult to review iron studies in infant as the study designs are very different, there is no uniform study protocol. But the available information suggests that iron supplementation should start early. This has been confirmed in a recent analysis of 3 studies (Jin H-X et al, 2015). All the studies suggested that early iron (2–3 weeks) reduced the need for blood transfusion. There were no data to suggest that iron increases the incidence of NEC.
We performed a randomized trial on early iron supplementation in infants < 1,300 g (Franz/Mihatsch, 2000). Early iron supplementation was associated with less transfusion requirement and less iron deficiency defined by low ferritin (Chart 2). This was also confirmed by a study with iron fortified human milk fortifier (Fe-HMF) in VLBW infants starting at 100 ml/kg/d milk intake (Berseth C et al, 2004). 14% of the Fefortified group required transfusion vs. 32% of the group with standard-HMF between days 15-28. In 2013 a study, similar to our study, performed on VLBW infants confirmed, that early enteral iron supplementation (4-6 mg/kg/d vs. 0–2mg/kg/d) reduced the number of transfused infants > d14 (Taylor / Kennedy, 2013). This is a significant beneficial effect.
There is a huge lack of data of early iron supplementation and long-term outcome. We did a follow-up at 5.5 years and we were able to have 85% of the survivors of our study.
The infants with early supplementation average Kaufmann IQ was 96 in contrast to 91 in infants with late supplementation (no significant difference; p=0.10). However, there was a significant difference with regard to gross motor function (normal function 81% vs. 65%). There- fore, there is a trend: early iron may be beneficial for the long-term outcome in VLBW infants. This data requires reconfirmation in further trials.
How much iron should be given?
The intrauterine iron accretion, referring back to the data of Widdowson, at a growth speed of 17 g/kg/d is 1,200 µg/kg/d. in addition Iron intake should compensate for phlebotomy loss 500 µg iron per ml blood. It has been suggested that a steady state absorption requirement should be 500–1000 µg/kg /d (Friel J, 1995). The critical problem is that iron absorption and incorporation into circulating erythrocytes are low and variable.
On average iron absorption is between 7%–10%, and at best at about 25%, has been reported in different studies. But all these studies together have been criticized in a review, because of methodology flaws (Fomon et al, 2000). So, iron retention in infants is largely unknown.
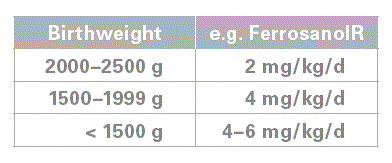
How much iron? In our study iron intake was 3 +/- 1 mg/kg/day (mean+/-SD). Therefore, we would recommend a dose of 2–4 mg/ kg/d. But we had infants who required higher doses than 4 mg/kg/ day to prevent iron deficiency. Therefore, the iron status needs to be monitored, e.g. using ferritin. In addition, measuring RetHb (>28 pg) may be a useful parameter as well.
In some infants’ intravenous iron administration has been shown to be effective and seemed to be safe, but there a no RCT on long-term outcome available (Friel JK, 1995; Mayer MP, 1996; Ohls RK, 1997).
Is enteral iron safe?
There is always the risk of iron toxicity
- Iron cannot be excreted from the body
- The anti-oxidative capacity of preterm infants is immature Free iron is toxic (oxidative stress)
- Fenton reaction (Fe2+ + H2O2 > Fe3+ + OH- + •OH)
- Formation of aggressive oxygen radicals, e.g. O2-, •OH, H2O2, ...
- Lipid-and protein peroxidation
- Promotion of ROP or BPD?
Does iron induce oxidative stress in infants?
There are quite a few studies which look at this topic.
- 8–16, 3–12, and 4 mg/kg/d p.o.
>> no effect (Braekke, 2007, Miller, 2006, Friel, 2005)
- 2mg/kg/d i.v.+ 9mg/kg/d p.o.
>> transient increase in malondialdehyde right after infusion (Pollak, 2001)
- 200µg/kg/day i.v.+ EPO
>> no effect on malondialdehyde/superoxide dismutase at 2 weeks (Qiao L, 2017)
Is there clinical evidence for iron toxicity?
- There is an association between multiple blood transfusions and
ROP (Inder J, 1997; Hesse, 1997)
CLD (Cooke EurJ Pediatr1997, Silvers ADC 1998).
- i.v. iron is associated with biochemical markers of lipid and protein peroxidation e.g. malondialdehyde (Pollak, 2001)
Which conclusion can we give for enteral iron supplementation?
No adverse effects in preterm infants have been observed with enteral supplementation.
- No free iron
- No membrane peroxidation marker
- No increase in ROP / CLD
(van Zoeren, 1998; Franz, 2000; Friel, 2001; Miller, 2006)
- No effect on growth, days off feeds, NEC …
(Franz, 2000; Berseth, 2004; Berglund, 2010; Taylor, 2013)
- Enteral iron reabsorption decreases right after blood transfusion (Dauncey, 1978)
Conclusion
Iron supplementation in preterm infants
Early appears to be safe and beneficial (Long 2012)
Start early (Long 2012, Lundström1977, Friel 1995, Franz 2000, ...)
At least 2–4 mg/kg/d p.o. (AAP, 1998, ESPGHAN, 2010)
Monitor iron status
Nutrition of preterm infants when returning home
The speaker: Umberto Simeoni, Switzerland
Post-discharge nutrition of preterm infants remains an ongoing debate, as intensive early nutrition, including after discharge nutrition, may offer positive effects on growth and development, at the cost of long term health alteration.
A significant proportion of very preterm infants still grow poorly during the postnatal period, while growth is dependent on nutrition and is importantly associated with brain development. Preterm birth is still associated with significant risks for altered growth and neurodevelopment, and increased risks of the metabolic syndrome, and other chronic, non-communicable diseases (NCDs) such as hypertension at adulthood (Modi N et al Pediatr Res 2011).
We observed a similar trend e.g. in blood pressure of young adults born preterm. At around the age of 20 years they had higher blood pressure, about 5–10 mm mercury higher, which is highly significant for a higher risk for stroke later in life. This higher blood pressure was correlated with the slope of catch-up growth in early in- fancy (Tauzin et al, JDOHaD 2014).
Avoiding extra-uterine growth restriction would avoid accelerated catch-up growth.
Early nutrition matters. Aside epidemiologic and clinical data, animal models demonstrate that even physiologic variations in nutritional intakes during development have consequences at adulthood. We observed that, in rodents, transient lactation overfeeding during the postnatal critical window of sensitivity, induced a significant increase in body fat mass. In addition, mice had a higher glucose concentration and glucose intolerance with insulin resistance.
Looking at the mechanisms of altered developmental programming and trying to understand where can the memory be between what happens very early in life and later on, we found in the liver of these animals marks of accelerated cellular senescence (Yzydorczyk et al., 2017).
Altered developmental programming, accelerated cellular senescence as intimate mechanisms of the long term effects of early nutrition.
This change was related to a deficit in SIRT1, sirtuins being a major family of proteins involved in the control of cellular senescence. Interestingly, in another study we observed a SIRT1 deficiency also in preterm born infants when studying the cardiovascular system, which is quite involved in the risk for long-time diseases (Frizeira- Vassallo et al, 2014).
SIRT1 decrease correlated with accelerated senescence of Endothelial Colony Forming Cells (ECFCs), which resulted in altered angiogenesis and a possible pathway towards later hypertension.
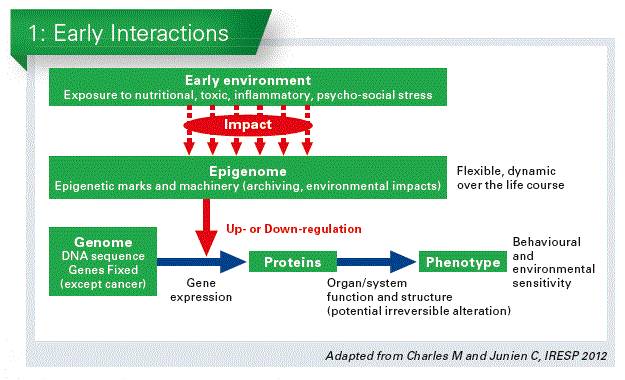
The memory behind early nutrition and what happens later in life and especially in preterm born infants seems due at least in part to epigenetic changes. The epigenome is virtually an additional layer to the genome, the expression of which it regulates. Biochemical modifications in the surface of the genome, such as methylation of DNA, or modifications of the histones, and nonc-coding RNAs (such as micro-RNAs) are involved. These modifications do not change the gene structure, they only up-or down-regulate the activity of a specific gene. What is important: this change is long-lasting. And it can even be inherited to the next generation – without being a genetic change. This may explain why we observed such altered function at adulthood in these babies (Chart 1).
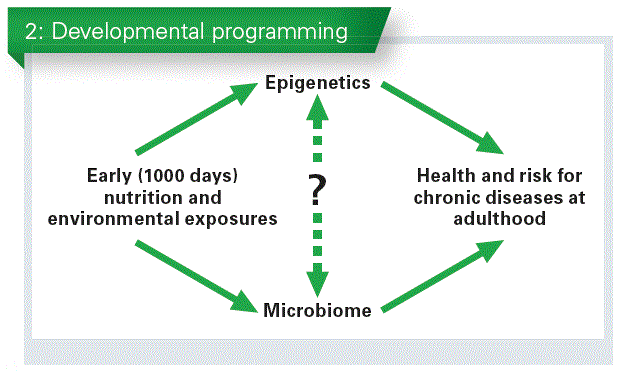
The microbiome has been also associated with what happens between the first 1000 days of life and health at adulthood (Chart 2).
What we need to know, is how both systems, the microbiome and the epigenome, combine their effects or which one intervenes before or after the other one, in other terms, to identify the complex relationship between the causes and the consequences.
Intensive early nutrition of preterm infants improves organs and systems function, growth and neuro-development, but may increase the risk for chronic disease in adulthood. Both objectives need to be achieved with an optimal early nutrition.
Which nutrition can be given after discharge?
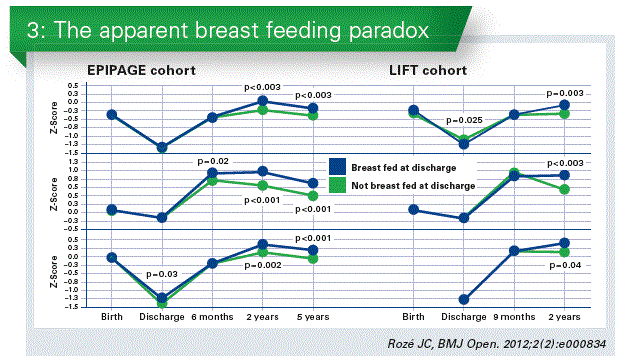
Own mother’s milk, or human milk are the preferred approaches and have a lot advantages for the preterm infant. Human breast milk reduces the rate of enterocolitis, of late sepsis. After discharge, breast fed preterm infants have less upper airway and gastro-intestinal infections, and are less prone to develop later type 1 diabetes and obesity. Neuro-development and brain deep gray matter volume are increased at the age of 7 years. Although breast feeding of preterm infants has limitations due to the high requirements of these infants for growth and development, especially in energy and proteins, in a study combining two separate cohorts, breastfed preterm babies who were breastfed grew paradoxically better than those who were not breastfed, although they received lower macro-nutrients intakes. In addition preterm babies who receive breast milk after discharge had a better neuro-development indexes compared to infants who do not receive it, as studied in two separate follow-up cohorts (Rozé JC, 2012) (Chart 3). Using a human milk fortifier to improve nutrients intake is an option. However, evidence is of moderate quality, due to inconsistency concerns between trials. Longer term follow-up of infant participants unfortunately did not confirm the persistance of such outcomes, as confirmed in a recent meta-analysis.
Formula feeding
Two types of formulas are available for post-discharge nutrition.
- Post-discharge formulas, which are moderately enriched in nutrients compared to term infants formulas.
- Preterm infant formulas, which have an even higher energy density and protein content, compared to post-discharge formulas.
A number of studies have been performed with very similar limitations. A recent meta-analysis showed no clear effect on weight gain for post-discharge formulas. To the contrary, preterm formulas benefit preterm born babies in terms of weight and head circumference growth, but not of neuro-development (Cochrane Database of Systematic Reviews, 2016).
Available data and knowledge on post-discharge nutrition currently fails to convince that they should be used for routine nutrition of preterm infants after discharge. Such formulas may however be of interest in specific subsets of patients, such as those who suffered fetal growth restriction and fail to achieve a normal growth around their term age. Preterm formulas do allow a better weight and length growth, however their long term benefit, especially for neuro-development, needs to be further investigated.
Whatever the feeding regimen of the baby, a rationale exists to accommodate nutrition in order to allow a linear growth in the corridor followed prenatally and postnatally by each individual patient. However, this would need highly powered randomized trials testing a combined nutritional strategy, associating early parenteral and enteral nutrition, followed by breast-or formula feeding.
Conclusion
Adequate, early nutrition is key to ensure brain development and neuro-development in preterm born subjects.
Preterm born subjects are exposed to an increased risk of chronic diseases at adulthood, in particular cardio-vascular, metabolic, neuro-cognitive and psychic disorders, likely due to missed adaptive developmental programming.
Poor intrauterine and extrauterine nutrition, excess late catch-up growth seem additional hits that may compromise the long term health of these subjects.
Targeting a linear, customized/personalized growth over the fetal and postnatal periods may be the appropriate strategy, including post-discharge nutrition (more evidence needed).
For such purpose, after hospital discharge and based on individual situations:
Breastfed infants may receive supplemental nutrients via a milk fortifier
Formula fed infants may receive a post-discharge or a preterm formula to achieve their individual growth potential
More research is needed to determine the optimal nutritional intakes whole strategy over infancy.