Water-Soluble Vitamins in Human Milk: Factors Affecting Their Concentration and Their Physiological Significance
Abstract
Most B vitamins and vitamin C are among the nutrients in milk most strongly affected by maternal status and/or dietary intake. Recent analytical methods are more efficient and valid, revealing major differences in water-soluble vitamins across population groups. An inadequate supply in milk can be detrimental to the breastfed infant’s health and development although cutoff points below which risk is increased are often uncertain, and little attention has been paid to adverse effects of low milk water-soluble vitamins on infant health and function. Concentrations change during lactation: thiamine, niacin, and pantothenic acid increase; B6, B12, and ascorbic acid gradually decrease; while riboflavin concentrations are stable, as is choline after an initial increase. Folate fluctuates until stabilizing in late lactation. Water-soluble vitamin concentrations in milk are also influenced by maternal supplementation, and, for some, by parity, preterm delivery, smoking, and maternal illness. However, there is relatively little change in concentrations during a feed nor is diurnal variation a major influence. Reported concentrations are used to set adequate intakes for infants and incremental requirements for lactation. However, the status of available data is poor due to the small number of participants in most studies, uncertainties about maternal nutritional status, and variable times of milk collection postpartum.
Introduction
All of the water-soluble vitamins are essential for the health, development, and survival of the breastfed infant. During the first 6 months of life, it is recommended that human milk is the sole source of nutrients for infants, and that it should remain an important source through at least 2 years of age. However, relatively little attention has been paid to the micronutrient content (quality) of milk from women consuming poor diets. Due in part to improvements in analytical methods, there is increasing evidence that the water-soluble vitamins are among the nutrients most likely to be secreted in reduced amounts if the moth- er’s status and/or intake are low.
In this article, for each of the water-soluble vitamins we summarize available information on the forms and concentrations in milk; the effects of poor maternal status on levels in milk and subsequent effects on infant status and function; normal changes during lactation; the effects of maternal interventions to increase concentrations in milk; and of other factors such as parity and smoking. We present comparative values for concentrations in milk from lower in- come countries measured by an efficient ultra-or high-performance liquid chromatography tandem mass method developed in our laboratory [1] and samples collected by collaborators from unsupplemented women at similar stages of lactation.
Much of the information presented is discussed in more detail in our recent series of 7 articles on micronutrients in human milk [2]. There, we discuss the paucity of data on milk micronutrients and the lack of reliable values for setting the adequate intakes (AIs) by the Institute of Medicine (IOM) and other countries.
Forms in Human Milk, Effects of Maternal Status, and Relationship to Infant Function
Thiamine
Thiamine (B1) and its phosphate esters are crucial for normal carbohydrate, nucleic acid, and amino acid metabolism, for example, decarboxylation of α-keto acids, the transketolase reaction in the pentose phosphate pathway, syn- thesis of the neurotransmitter acetylcholine, and nerve impulse transmission. The predominant forms in human milk are thiamine monophosphate (∼60%) and free thiamine (∼30%), plus a small amount of thiamine triphosphate. The global prevalence of thiamine deficiency is uncertain but is likely high in populations where grains are polished and animal source food intake is low. For example, in a nationally representative sample from the 2014 Cambodian National Micronutrient Survey, at 6–12 months postpartum, 27% of mothers and 38% of infants were thiamine deficient [3]. Maternal deficiency is rapidly reflected in low milk thiamine, with concentrations <0.12 mg/L associated with poor infant growth in an older Malaysian study. Based on analyses in our laboratory, we find that in Cambodia, Ghana, India, the Gambia, Indonesia, and Peru, milk concentrations are only 60–70% of the 0.21 mg/L IOM-accepted value, which was based on a 1985 report. Beriberi presents in breastfeeding infants who are thiamine deficient due to severe maternal deficiency, but it is much less common than 50 years ago. However, even in infants with marginal status, mortality peaks at age 3 months [3], and growth and auditory, motor, and language development was retarded years later in Israeli children who consumed formula lacking the vitamin when they were infants [4]. A 2004 study reported that thiamine deficiency was common among breastfeeding sick infants in Laos and associated with a higher risk of mortality [5].
Riboflavin
As part of the coenzymes flavin adenine mononucleotide and flavin adenine dinucleotide (FAD), riboflavin (B2) is required for energy production, fatty acid and amino acid synthesis, DNA repair, folic acid activation, conversion of hepatic tryptophan into niacin, and production of glutathione, a free radical scavenger. Humans need a constant dietary supply of vitamin B2 (from meat, milk, and dairy intake) as it is inefficiently stored. The predominant forms in human milk are FAD (60%), free riboflavin (30%) and other flavin derivatives [6]. A concentration of 0.35 mg/L was accepted by the IOM for setting the infant AI although this was based on samples from only 5 mothers. Milk riboflavin con- centrations are highly dependent on maternal intake of the vitamin [7]. Mater- nal intake and status are strongly dependent on usual intake of dairy products, although studies of the global prevalence of inadequacy are relatively few. About 75% of young women and 95% of adolescent girls had poor riboflavin status in the UK diet survey (mostly low dairy product consumers), and the prevalence of deficiency was reported to be high in Canada, Europe, and in an Irish Na- tional Survey [8]. In pregnant and lactating women in Guatemala, Nepal, and India, riboflavin deficiency occurred in 77–85% although these were older data and not nationally representative. Our analyses show concentrations in milk are only 10–20% of the values used to set the AI in samples from Bangladesh, Kenya, Peru, Cambodia, Indonesia, and the Philippines. Poor riboflavin status has been reported in an older study of Gambian infants whose mother’s milk was low in the vitamin [9], but the effects of deficiency on infant development and status are not known.
Niacin
Niacin (B3) is the collective term for the interconvertible nicotinic acid and nicotinamide, the building blocks for nicotinamide adenine dinucleotide (NAD) and nicotinamide adenine dinucleotide phosphate (NADP). These coenzymes are involved in oxidation-reduction reactions (e.g., electron carriers for intracellular respiration), catalyze the oxidation of fuel molecules as codehydrogenases (NAD), or serve as a hydrogen donor in the reductive processes of fatty acid synthesis (NADP). Humans are capable of hepatic in vivo nicotinic acid production from tryptophan where 60 mg produces 1 mg niacin. This ratio is not certain for the infant. Forms of niacin in human milk include nicotinamide, NAD, NADP, nicotinamide riboside, and nicotinamide mononucleotide. The global prevalence of niacin deficiency is not known although it is likely to be greater where maize is not lime treated, or the staple diet is starchy, low-protein foods. The IOM accepts 1.8 mg/L as the normal milk value, but this is based on one old UK study including 23 women from 16 to 244 days lactation, where the range was 1.2–2.8 mg/L. We find that values are <20% of this concentration in California, Cambodia, Indonesia, Bangladesh, the Philippines, India, Peru, Malawi, and Kenya. Surprisingly, concentrations were highest in Ghana and the Gambia, where they approached 60% of the AI value.
Pyridoxine
Vitamin B6 (pyridoxal, pyridoxine, and pyridoxamine) is a cofactor for >100 enzymes required for amino acid metabolism, glucose synthesis, glycogen break- down by phosphorolysis, and steroid (and other nuclear-acting) hormone regulation. The form in milk is mainly pyridoxal (75%) with small amounts of pyridoxal phosphate, pyridoxamine, and pyridoxine. Concentrations of B6 vitamers in milk are positively correlated with maternal status based on the assessment of plasma pyridoxal 5′-phosphate (or PLP) [10]. The global prevalence of deficiency in women is unknown. It has been reported as high in US and Canadian surveys although there is uncertainty about the cutoff point that should be used. Older studies reported that 40% of Egyptian mothers had milk B6 <0.1 mg/L as- sociated with lower birth weight, abnormal infant behavior, and less responsivity in the mothers [11]. About 15% of exclusively breastfed Finnish infants had poor B6 status at age 6 months associated with slower growth. In the USA, although mothers consumed the recommended dietary allowance (RDA) for B6, those with higher intakes had more in their milk and their infants had higher scores on the Brazelton Neonatal Behavioral Assessment Scale [12]. The IOM reported a value of 0.13 mg/L from 19 participants at 3 weeks to 30 months postpartum. We find concentrations <60% of this value in samples from Cambodia, Bangladesh, Peru, Indonesia, the Philippines, Ghana, and the Gambia. Interestingly concentrations in a Californian group of lactating women were 230% of the AI, possibly because vitamin B6 is included in relatively high amounts in many prenatal supplements.
Cobalamin (B12)
B12 in biological materials is predominantly present as coenzyme B12, in which the axial group is either occupied by a methyl group or an adenine nucleoside connected by a cobalt-carbon. These forms are important enzymes in the folate-dependent methylation of homocysteine to methionine and the conversion of methylmalonyl-coenzyme A to succinyl-coenzyme A (1-carbon metabolism), and DNA synthesis. The vitamin is tightly bound to the binding protein apohaptocorrin in milk, which can interfere with cobalamin measurement; this problem has been avoided in recent assays [13]. In human milk, vitamin B12 is mainly present as methylcobalamin and 5′-de- oxyadenosylcobalamin with small contributions of hydroxo- and cyanoco- balamin. The IOM accepted a concentration of 0.42 μg/L based on a small sample of Brazilian women and possibly invalid analytical methods, but concentrations in a small group of Californian and Danish samples were close to the AI in the more recent report. Analyses in our laboratory reveal milk B12 concentrations ranging from about 20% (Kenya, Guatemala) to 50% of the AI in most low-income countries, but AI values were higher in Malawi (60%), Bangladesh (80%), and Ghana (120%), possibly due to the consumption of small dried fish.
It has long been established that maternal vitamin B12 deficiency caused by strict vegetarianism or undiagnosed pernicious anemia produces symptoms of deficiency in exclusively breastfed infants around age 3–4 months. These include growth stunting and severely reduced head circumference, apathy, inability to consume complementary foods, and motor and cognitive delays which do not completely resolve in about 50% of cases after intramuscular or high- dose treatment [14]. Milk B12 is lower even in marginally depleted mothers, and there is a high prevalence of marginal B12 deficiency in the many populations that consume low amounts of animal source foods. A positive correlation be- tween maternal intake of B12 and milk B12 has been demonstrated in several countries including Kenya, Guatemala, and Mexico. B12 behaves somewhat differently from other B vitamins in that infant status may depend more on the accumulation of liver B12 stores in utero than on maternal intake during pregnancy or lactation. For example, even when B12-depleted Bangladeshi mothers were given a relatively high dose (250 μg/day) from 18 weeks of pregnancy through 3 months of lactation, infant status at 3 months was still correlated with maternal serum B12 in early pregnancy [15]. This suggests a long-term effect of maternal status on infant status probably via in utero accumulation of the vitamin in fetal liver. The remaining challenge is to document if there is a causal link between marginal maternal B12 status, milk B12, and impaired infant development.
Folate
Folate in its coenzymatic form is needed for the transfer of 1-carbon atom groups such as formyl (CHO), methyl (CH3) or formimino-functions (CH=NH) in amino acid metabolism and in purine and pyrimidine synthesis in nucleic acid formation (DNA and RNA). The main form in milk is N- 5-methyl tetrahydrofolate. The IOM accepts 85 μg/L as the average milk folate concentration. Folate behaves differently from the other B vitamins in that the mammary gland maintains the concentrations in milk at the expense of ma- ternal stores [16], thus neither maternal intake nor status is related to milk folate concentration.
Biotin
Biotin is a component of carboxylase enzymes that are vital for amino acid metabolism, gluconeogenesis, fatty acid biosynthesis, and odd-chain fatty acid catabolism. Forms in milk include biotin and its metabolites bisnorbiotin and biotin sulfoxide. In the genetic condition BIOT, lack of biotinidase leads to biotin deficiency resulting in symptoms such as seizures, hypotonia, and respiratory problems within a few months of birth. However, biotin is ubiquitous in the diet, and there are no reports of low biotin in milk due to maternal deficiency.
Choline
Choline is a precursor for the neurotransmitter acetylcholine and for betaine. As a coenzyme, choline is vital for the structural integrity of cell membranes, methyl metabolism, cholinergic neurotransmission, transmembrane signaling, and lipid and cholesterol transport and metabolism. Human requirements for choline are relatively large, and the IOM accepts an estimate of 160 mg/L milk from the two available studies. The main forms in milk are water-soluble free choline, phosphocholine, and glycerophosphocholine, with little contribution (∼10%) of fat-soluble phosphatidylcholine (lecithin) and sphingomyelin. Maternal serum choline is correlated with milk and infant concentrations, and milk choline is also affected by MTHFR genotype [17]. It is likely that adequate milk choline is vital for the normal development of the infant based on the fact that large amounts of its oxidation product, betaine, are excreted during the first year, and a lower choline status of young children has been associated with stunting [18].
Ascorbic Acid (Vitamin C)
This water-soluble, antioxidant electron donor supports the immune system, stimulating leukocytes, increasing antibody production, and stimulating production of interferons. It is required for hydroxylation of amino acids in collagen. In milk, the main forms are ascorbic acid and dehydroascorbic acid, totaling an estimated 1.8 mg/L in the three studies used by the IOM to set the AI. Low intakes of this vitamin are common where fruit and vegetable availability fluctuate by season, so milk concentrations may be greatly seasonally affected [19]. Consuming a rice and lentil complementary food with human milk to increase ascorbic acid intake did not improve the inhibitory effect of phytate on iron absorption by Bangladeshi infants and young children. Adverse effects of low milk ascorbic acid on the infant have not been reported.
Concentration Changes during Lactation
Concentrations of all water-soluble vitamins occur during the postpartum period, as summarized elsewhere by Dror and Allen [20]. Thiamine, niacin, and pantothenic acid concentrations in milk increase throughout the first few months of lactation. Riboflavin concentrations are fairly constant in well-nourished mothers within at least the first 3 months of lactation. Indian women of low socioeconomic status showed increasing riboflavin concentrations to a peak at 2–4 months and a decrease by 5–6 months [21]. Similarly, vitamin B6 concentrations increase 300–400% during the first weeks postpartum and fall later in lactation, raising concern that human milk alone may be insufficient to meet the infant’s requirements at 6 months of age [22].
A systematic review of studies using valid methods of B12 analysis indicate that B12 concentrations are very high in the colostrum and then fall in the first few weeks postpartum and are stable until about 2–4 months [23]. In well- nourished Danish mothers, B12 concentrations were higher at 9 months than they were at 4 months, primarily due to an increase in haptocorrin [24]. Also, in well-nourished Danish and Norwegian women, infant plasma B12 decreased and methylmalonic acid increased inversely with milk B12 concentration, such that the ability to supply sufficient B12 to infants in mid-lactation has been questioned, even for well-nourished mothers [25]. Formula-fed infants usually have much higher serum B12 concentrations than those exclusively breastfed.
Unlike the situation for most B vitamins, folate is low in colostrum, increases during the next few weeks, peaks at 2–3 months, falls between 3–6 months, and then is stable until late lactation [26]. Total choline also increases between 7 and 22 days after delivery and then remains unchanged in mature milk; however, free choline falls between 12 and 180 days [27]. Concentrations of vitamin C are highest in colostrum and fall during lactation [26].
Effects of Maternal Supplementation or Food Fortification
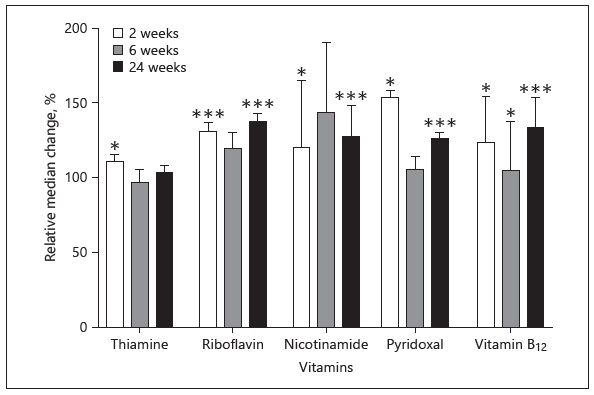
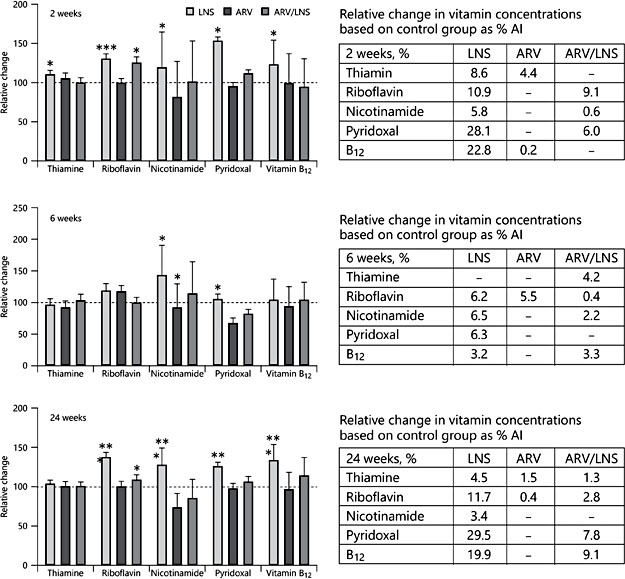
The B vitamins differ in the extent and rate at which they increase in milk in response to maternal supplementation. The effects of acute supplementation were studied in Bangladeshi women who received a single dose of ∼1× the RDA of multiple micronutrients followed next day with a single dose of 2× RDA before breakfast. Concentrations of vitamins were measured in milk at each feed during the next 24 h [28]. Acute responses were observed for thiamine, riboflavin, and pyridoxal. Although only about 1% of the supplemental vitamins were secreted into the milk, this amount was equivalent to 2–99% of the AI for infants aged 0–6 months. Maternal supplementation with micronutrients for 6 months likewise showed that only a few percent of the supplemented water-soluble vitamins appeared in the milk [29] (Fig. 1). In this study, conducted in Malawi, HIV- positive mothers were enrolled to evaluate the impact of antiretroviral therapy and micronutrient supplements on maternal morbidity and HIV transmission in breast milk. The lipid-based micronutrient supplement provided daily within a few days of delivery increased milk thiamine, riboflavin, nicotinamide, B6, and B12 within 2 weeks (Fig. 1). Interestingly, concentration had not increased further at 24 weeks. ARV treatment eliminated the positive effects of lipid-based micronutrient supplements on milk B vitamins.
Studies have also evaluated the impact of single B vitamins on the amounts secreted in milk. In the case of thiamine, a rapid response was reported in population groups with a high prevalence of deficiency. In a small trial in thiamine- deficient Cambodian women, a dose of 100 mg for 5 days increased milk thiamine from 180 (85–359) to 503 (360–808) nmol/L on day 6 [30]. An intervention that supplied Cambodian mothers with poor status a thiamine-fortified fish sauce for 6 months, starting in pregnancy, increased thiamine in milk from 144 to 207 μg/L 4 months postpartum and also improved infant thiamine status [31]. In general, maternal supplementation has a positive effect on milk riboflavin concentration [32]. Maternal supplementation of deficient mothers in the Gambia improved their status, milk riboflavin, and infant status [9]. B6 supplementation also increases milk concentrations within hours [33], and there was a dose response when mothers were supplemented with pyridoxine hydrochloride from 0 to 20 mg for 3 days [34]. Phosphatidyl choline supplements increase milk free choline and phosphocholine [17]. In Canada, maternal folic acid supplementation, especially if >400 μg/day, increased folate in milk by 18% and free folic acid by 126%, and reduced 5-methyl tetrahydrofolate by 19% [35].
More maternal supplementation studies have provided B12 in lactation compared to other B vitamins, due to the well-documented severe and potential irreversible symptoms of deficiency that occur in the exclusively breastfed infants of deficient mothers. A recent systematic review showed that supplementation of mothers during pregnancy and early lactation, or during months 0–6 of lactation, increased milk B12 significantly compared to placebo or non-placebo controls [23]. Doses were 50 μg/day in pregnancy and the first 3 months of lactation, or 3–1,000 μg/day during lactation. There was little difference in the level of milk response across this range of doses, probably due to the well-known inverse relationship between the efficiency of active absorption and B12 dose. Among all the intervention studies conducted to date, the most remarkable increase in milk B12 occurred in Cameroon within 1 year of a wheat flour fortification program [36], possibly reflecting more efficient absorption of consistent low doses of the vitamin.
Other Factors
Other factors that can affect water-soluble vitamins in milk include parity, preterm delivery, diurnal variation, smoking, and illness, although these effects have not been studied systematically, and available information is patchy, as summarized elsewhere [20]. There is virtually no information on how any of these factors affect levels of thiamine. Parity does not affect milk riboflavin or folate. Both higher and lower B6 concentrations have been reported in preterm milk. In the two studies examining whether preterm delivery affects B12 in colostrum, transitional milk, or preterm milk, there was no relationship. Choline is lower in preterm milk whereas vitamin C is more concentrated. Choline in milk is related to maternal inflammation, prolactin (positively), and cortisol (negatively). Smoking reduces milk vitamin C.While significant yet small differences were found for concentrations of thiamine, riboflavin, and nicotinamide based on timing of collection during a feeding, pyridoxal and B12 were unaffected. Diurnal variability is small compared to interindividual variation in concentrations. The best time of collection for reflecting average daily concentrations was observed during the afternoon, but maternal supplementation can affect this natural fluctuation [28]. Milk folate is higher in the afternoon and evening than in the morning.
Fig. 1. Mean (SEM) concentrations of water-soluble vitamins in human milk 2, 6, and 24 weeks after maternal multiple micronutrient supplementation and/or ARV treatment. The 100% reference line is the value in nonsupplemented women at each time point. * p < 0.05, *** p < 0.001, control vs. supplemented groups. Women received daily lipid-based nutrient (LNS) supplementation and/or antiretroviral (ARV) treatment for 24 weeks post- partum. AI, adequate intakes. Modified from Allen et al. [29].
Conclusions
Water-soluble vitamins in human milk have been measured in only a small number of studies, and the validity of the values used to set the intake recommendations for infants and lactating women is uncertain. However, it is clear that the milk concentrations of these vitamins in all the low- and middle-income countries we have measured are substantially lower than the values used to set the AIs – in some countries substantially so. The global prevalence of poor water-soluble vitamin status is almost unknown, as are the effects of low milk concentrations on infant growth, function, and development. We also need to learn the most effective ways of improving maternal, milk, and infant status including the timing of supplements and the effectiveness of food fortification. Currently, we are conducting a 4-country study to establish reference values for micro-nutrients in milk from well-nourished women across lactation and their relationship to maternal diet and maternal and infant status indicators.
References
- 1 Hampel D, York ER, Allen LH: Ultra-performance liquid chromatography tandem mass- spectrometry (UPLC-MS/MS] for the rapid, simultaneous analysis of thiamin, riboflavin, flavin adenine dinucleotide, nicotinamide and pyridoxal in human milk. J Chromatogr B Analyt Technol Biomed Life Sci 2012;903: 7–13.
- 2 Allen LH, Dror DK: Introduction to current knowledge on micronutrients in human milk: adequacy, analysis and need for research. Adv Nutr 2018;9(suppl 1):275S–277S.
- 3 Whitfield KC, Karakochuk CD, Liu Y, et al: Poor thiamin and riboflavin status is common among women of childbearing age in rural and urban Cambodia. J Nutr 2015;145: 628–633.
- 4 Mimouni-Bloch A, Goldberg-Stern H, Strausberg R, et al: Thiamine deficiency in infancy: long-term follow-up. Pediatr Neurol 2014;51:311–316.
- 5 Khounnorath S, Chamberlain K, Taylor AM, et al: Clinically unapparent infantile thiamin deficiency in Vientiane, Laos. PLoS Negl Trop Dis 2011;5:e969.
- 6 Roughead ZK, McCormick DB: Flavin composition of human milk. Am J Clin Nutr 1990;52:854–857.
- 7 Kodentsova VM, Vrzhesinskaya OA: Evaluation of the vitamin status in nursing women by vitamin content in breast milk. Bull Exp Biol Med 2006;141:323–327.
- 8 Powers HJ, Hill MH, Mushtaq S, et al: Correcting a marginal riboflavin deficiency improves hematologic status in young women in the United Kingdom (RIBOFEM). Am J Clin Nutr 2011;93:1274–1284.
- 9 Bates CJ, Prentice AM, Watkinson M, et al: Riboflavin requirements of lactating Gambian women: a controlled supplementation trial. Am J Clin Nutr 1982;35:701–709.
- 10 Chang SJ, Kirksey A: Pyridoxine supplementation of lactating mothers: relation to maternal nutrition status and vitamin B-6 concentrations in milk. Am J Clin Nutr 1990;51: 826–831.
- 11 McCullough AL, Kirksey A, Wachs TD, et al: Vitamin B-6 status of Egyptian mothers: relation to infant behavior and maternal-infant interactions. Am J Clin Nutr 1990;51:1067– 1074.
- 12 Ooylan LM, Hart S, Porter KB, et al: Vitamin B-6 content of breast milk and neonatal behavioral functioning. J Am Diet Assoc 2002; 102:1433–1438.
- 13 Hampel D, Shahab-Ferdows S, Domek JM, et al: Competitive chemiluminescent enzyme immunoassay for vitamin B12 analysis in human milk. Food Chem 2014;153:60–65.
- 14 Dror DK, Allen LH: Effect of vitamin B12 deficiency on neurodevelopment in infants: current knowledge and possible mechanisms. Nutr Rev 2008;66:250–255.
- 15 Siddiqua TJ, Ahmad SM, Ahsan KB, et al: Vitamin B12 supplementation during preg- nancy and postpartum improves B12 status of both mothers and infants but vaccine response in mothers only: a randomized clinical trial in Bangladesh. Eur J Nutr 2016;55: 281–293.
- 16 Mackey AD, Picciano MF: Maternal folate status during extended lactation and the effect of supplemental folic acid. Am J Clin Nutr 1999;69:285–292.
- 17 Fischer LM, da Costa KA, Galanko J, et al: Choline intake and genetic polymorphisms influence choline metabolite concentrations in human breast milk and plasma. Am J Clin Nutr 2010;92:336–346.
- 18 Semba RD, Shardell M, Sakr Ashour FA, et al: Child stunting is associated with low circulating essential amino acids. EBioMedicine 2016;6:246–252.
- 19 Bates CJ, Prentice AM, Prentice A, et al: Seasonal variations in ascorbic acid status and breast milk ascorbic acid levels in rural Gambian women in relation to dietary intake. Trans R Soc Trop Med Hyg 1982;76:341–347.
- 20 Dror DK, Allen LH: Overview of nutrients in breast milk. Adv Nutr 2018;9(suppl 1):278S– 94S.
21 Bamji MS, Chowdhury N, Ramalakshmi BA, et al: Enzymatic evaluation of riboflavin status of infants. Eur J Clin Nutr 1991;45:309– 313.
- 22 Heiskanen K, Siimes MA, Perheentupa J, et al: Risk of low vitamin B6 status in infants breast-fed exclusively beyond six months. J Pediatr Gastroenterol Nutr 1996;23:38–44.
- 23 Dror DK, Allen LH: Vitamin B-12 in human milk: a systematic review. Adv Nutr 2018; 9(suppl 1):358S–366S.
- 24 Greibe E, Lildballe DL, Streym S, et al: Cobalamin and haptocorrin in human milk and cobalamin-related variables in mother and child: a 9-mo longitudinal study. Am J Clin Nutr 2013;98:389–395.
- 25 Bjorke-Monsen AL, Torsvik I, Saetran H, et al: Common metabolic profile in infants indicating impaired cobalamin status responds to cobalamin supplementation. Pediatrics 2008; 122:83–91.
- 26 Karra MV, Udipi SA, Kirksey A, et al: Chang- es in specific nutrients in breast milk during extended lactation. Am J Clin Nutr 1986;43: 495–503.
- 27 Ilcol YO, Ozbek R, Hamurtekin E, et al: Choline status in newborns, infants, children, breast-feeding women, breast-fed infants and human breast milk. J Nutr Biochem 2005;16: 489–499.
- 28 Hampel D, Shahab-Ferdows S, Islam MM, et al: Variability of vitamin concentrations in human milk due to time within-feed, circadian rhythm and single-dose maternal supplementation. J Nutr 2017;147:603–611.
- 29 Allen LH, Hampel D, Shahab-Ferdows S, et al: Antiretroviral therapy provided to HIV- infected Malawian women in a randomized trial diminishes the positive effects of lipid- based nutrient supplements on breast-milk B vitamins. Am J Clin Nutr 2015;102:1468– 1474.
- 30 Coats D, Frank EL, Reid JM, et al: Thiamine pharmacokinetics in Cambodian mothers and their breastfed infants. Am J Clin Nutr 2013;98:839–844.
- 31 Whitfield KC, Karakochuk CD, Kroeun H, et al: Household consumption of thiamin fortified fish sauce increases erythrocyte thiamin concentrations among rural Cambodian women and their children younger than 5 years of age: a randomized controlled efficacy trial. J Pediatr 2017;181:242–247.e2.
- 32 Nail PA, Thomas MR, Eakin R: The effect of thiamin and riboflavin supplementation on the level of those vitamins in human breast milk and urine. Am J Clin Nutr 1980;33:198– 204.
- 33 Hamaker BR, Kirksey A, Borschel MW: Dis- tribution of B-6 vitamers in human milk during a 24-h period after oral supplementation with different amounts of pyridoxine. Am J Clin Nutr 1990;51:1062–1066.
- 34 Styslinger L, Kirksey A: Effects of different levels of vitamin B-6 supplementation on vitamin B-6 concentrations in human milk and vitamin B-6 intakes of breastfed infants. Am J Clin Nutr 1985;41:21–31.
- 35 Page R, Robichaud A, Arbuckle TE, et al: Total folate and unmetabolized folic acid in the breast milk of a cross-section of Canadian women. Am J Clin Nutr 2017;105:1101–1109.
- 36 Engle-Stone R, Nankap M, Ndjebayi AO, et al: Iron, zinc, folate, and vitamin B-12 status increased among women and children in Yaounde and Douala, Cameroon, 1 year after introducing fortified wheat flour. J Nutr 2017;147:1426–1436.