The Role of Hydrolyzed Formula in Allergy Prevention
Key Messages
- Breastfeeding is the optimal way to feed infants and therefore is recommended for all infants regardless of allergy risk.
- For those infants who are exposed to infant formula, some studies suggest that specific partially hydrolyzed or extensively hydrolyzed formulas may decrease the risk of eczema compared to nonhydrolyzed formulas for children with a family history of atopic disease.
- The literature to support the preventive effects of hydrolyzed infant formulas for asthma, allergic rhinitis, and food allergy is inconsistent and insufficient.
- The qualitative changes to the peptides by the method of hydrolysis, not just the degree of protein hydrolysis, may have a large influence on the preventive effect of a particular infant formula for the potential risk of allergic disease.
Key Words
Eczema · Atopic dermatitis · Asthma · Food allergy · Whey · Casein · Allergic rhinitis · Hay fever · Prevention
Abstract
Asthma, eczema, food allergy, and allergic rhinitis are some of the most common pediatric, chronic conditions in the world. Breastfeeding is the optimal way to feed all infants. For those infants who are exposed to infant formula, some studies suggest that certain partially hydrolyzed or extensively hydrolyzed formulas may decrease the risk of allergic disease compared to nonhydrolyzed formulas for children with a family history of atopic disease. Overall, there is some evidence to suggest that partially hydrolyzed whey formulas and extensively hydrolyzed casein formulas may decrease the risk of developing eczema for infants at high risk of allergic disease. The evidence for a preventive effect of hydrolyzed formulas on allergic rhinitis, food allergy, and asthma is inconsistent and insufficient. Finally, the qualitative changes to the peptides by the method of hydrolysis, not just the degree of protein hydrolysis, may have a large influence on the preventive effect of a particular infant formula for the potential risk of allergic disease. As a result, it may be difficult to generalize findings from clinical studies using a specific infant formula to other infant formulas from different manufacturers using different methods of hydrolysis. Further clinical studies are needed to help clinicians identify which infants may benefit from early intervention, as well as which specific hydrolyzed formulas are best suited to decrease the risk of future allergic disease.
Introduction
Asthma, eczema, food allergy, and allergic rhinitis are some of the most common pediatric, chronic conditions in the world. Although mortality from such conditions is relatively rare, there is great impact on healthcare utilization, missed days of work and school, as well as effects on quality of life, for both parents and children. There are currently many treatment strategies for each of these conditions. For example, the use of infant formulas with hydrolyzed proteins is commonly used to treat cow’s milk protein allergy.
Breastfeeding is the optimal way to feed all infants, whether at risk of allergy or not
From a broader, public health perspective, a preventive approach towards these chronic conditions would be more cost-effective and impact a large percentage of the population. Breastfeeding is the optimal way to feed all infants, whether at risk of allergy or not. For those infants who are exposed to infant formula, some studies suggest that hydrolyzed formulas may decrease the risk of allergic disease compared to nonhydrolyzed formulas. This article will review the current evidence regarding the role of hydrolyzed formula in allergy prevention, specifically for eczema, food allergy, asthma, and allergic rhinitis.
Hydrolyzed Infant Formulas
In the United States, the Federal Food, Drug, and Cosmetic Act defines an infant formula as “a food which purports to be or is represented for special dietary use solely as a food for infants by reason of its simulation of human milk or its suitability as a complete or partial substitute for human milk” [1]. Although human breastmilk is ideal, a large percentage of infants are exposed to formula in the first year of life. Based on 2013 data, 81% of newborns in the United States initiate breastfeeding; however, by 6 months of age, breastfeeding rates are 52%. By 12 months of age, this percentage drops to 31% [2]. Worldwide, infant formula exposure may be higher as the rates of breastfeeding may be lower. In low- and middle-income countries worldwide, only half of infants younger than 1 month are breastfed. This percentage falls to approximately 30% at 1–5 months of age [3]. As a result, a large percentage of children are exposed to infant formula at an early age.
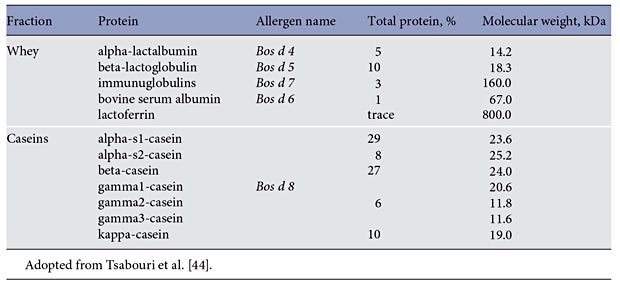
Infant formulas have been developed to mimic human breast milk. Typical infant formulas for full-term infants have 19–20 calories per ounce and approximately 1.3– 1.4 g of protein per 100 mL [4]. Although there are a variety of potential protein sources for infants, the typical protein source is cow’s milk proteins. Cow’s milk proteins can be separated into 2 general groups, casein and whey. Biochemically, the separation of these 2 proteins can be visualized when cow’s milk is acidified or exposed to chymosin (rennin). The casein and whey proteins are present in a 4 to 1 ratio and the specific proteins are listed on Table 1.
Infant formulas can be further classified as intact (or nonhydrolyzed), partially hydrolyzed formulas (pHF), extensively hydrolyzed formulas (eHF), or amino acid formulas. The current classification of infant formula focuses on the degree of hydrolysis; however, different manufacturers employ a number of different proprietary methods of hydrolysis. Using a variety of artificial methods, these intact proteins can be broken down into smaller components or peptides. In general, pHFs have peptides which are <5 kDa with a size distribution of 3–10 kDa, while eHFs have peptides which are <3 kDa [5]. Amino acid-based formulas contain free amino acids for infants who are sensitive to even small peptides of cow’s milk protein.
Potential Mechanisms of Action
When these proteins are consumed and enter the gastrointestinal tract, they are exposed to gut-associated lymphoid tissue (GALT), a key component of the mucosal immune system and an extensive immune organ. Due to its proximity to food antigen and the microbiome, GALT must continually be able to distinguish nonpathogenic from pathogenic organisms, as well as enable oral tolerance to specific food antigens [6]. The induction of oral tolerance seems to depend on the timing and the type of exposure [7]. The exposure of the smaller peptides to GALT is thought to induce oral tolerance without sensitization, as the decreased molecular weight has been associated with the decreased allergenicity of the protein. As a result, hydrolyzed formulas may decrease the risk of allergic disease compared to nonhydrolyzed formulas.
The relationship between the allergenicity of infant formulas based on the type of protein and degree of hydrolysis is most likely incomplete. Although this quantitative description is a useful starting point, the qualitative changes to the peptides by the method of hydrolysis may also play a large effect on the potential risk of allergic disease. For example, Lambers et al. [8] described the use of a combination of mass spectrometry-based peptidomics and multivariate clustering analyses to create a comprehensive analysis of different hydrolyzed milk protein formulas at the peptide level. The characterization of the specific peptide profiles of an infant formula may provide a better understanding of the likelihood of allergenicity. At least 3 factors, protein source, method of hydrolysis, and degree of hydrolysis, may influence the potential benefit of a hydrolyzed formula in allergy prevention. These observations may help explain why the degree of hydrolysis does not always correlate with the results of clinical trials comparing the effectiveness of pHF with that of eHF, or the lack of consistency of findings within classes of formulas based on the degree of hydrolysis.
Methodologic Issues in Evaluating the Literature
Although double-blind, randomized controlled trials are the gold standard to assess if hydrolyzed formulas decrease the risk of allergic disease, there are several methodologic issues to consider when reviewing and comparing results of studies from across the literature. The main comparison should be breastmilk and breastfeeding; however, it would be unethical to randomize infants into a situation where they were prevented from breastfeeding. In addition to the impossibility of blinding, there is also the issue that the composition of breastmilk differs from mother to mother [9]. As a result, most clinical trials will compare one type of formula versus another type of formula among infants who are not able to breastfeed for various reasons. In addition, if formula exposure is occurring during weaning from breastfeeding or being combined with breastfeeding, the extent of formula exposure may be difficult to control.
There are additional issues of heterogeneity in study design. To increase the likelihood of detecting an effect, studies may only recruit those infants at high risk of allergy based on family history. However, the extent of allergic disease in a family history can vary (e.g., number of relatives affected) and baseline risk can be difficult to determine [10]. In some instances, the use of infant formula is part of a larger environmental intervention. As a result, it is difficult to assess the contributing effect of infant formula exposure. Associated with this issue is the fact that the time to the development of the clinical outcome may be protracted. For example, asthma and allergic rhinitis can be difficult to confirm in a child <5 years of age. During the study period, other environmental, dietary, or medical access factors may confound the association between the exposure and the outcome. A combination of environmental and genetic factors likely plays significant roles in the pathogenesis of allergic disease [11].
Hydrolyzed Formulas and Primary Eczema Prevention
Eczema is a chronic skin disease characterized by pruritic, inflamed skin. It is the most common chronic skin disease in children, affecting approximately 20% of infants and young children [12]. In developed countries, the incidence of eczema has steadily increased [13]. Studies have shown a reduced in cidence of eczema among infants who are exclusively breastfed [14]. For infants who are not breast fed, cow’s milk protein is a common food allergen associated with the development of eczema. It has also been clinically observed that hydrolyzed formulas, used for the treatment of cow’s milk protein allergy, have been associated with decreased eczema. Specifically, the chemical and enzymatic hydrolysis reduces the molecular weight and the peptide size of cow’s milk protein and can decrease potentially sensitizing allergenic determinants.
Studies have shown a reduced incidence of eczema among infants who are exclusively breastfed
Several dozen studies have assessed the effectiveness of early exposure to hydrolyzed formula to decrease the risk of eczema. Two different meta-analyses, both published in 2010, suggest that healthy infants with a family history of allergy who are fed with partially hydrolyzed whey protein (pHF-W) formula have a reduced risk of atopic dermatitis compared with infants fed intact cow’s milk protein formula (CMF). Subanalyses conducted in metaanalyses by Szajewska and Horvath [15] and Alexander and Cabana [16] estimate that the risk reduction is 52 and 55%, respectively, at 12 months of age, and 38 and 36%, respectively, at the age of >30 months.
Since the publication of these meta-analyses, more recent analyses have been published that both support and do not support the effectiveness of hydrolyzed formula for eczema prevention. On the negative side, Lowe et al. [17] reported the results of a single-blind (participant) randomized controlled trial that compared allergic outcomes in 620 infants fed CMF, pHF-W, or soy formula at the cessation of breastfeeding. There was no difference in the development of eczema within the first 2 years of life for pHF-W (odds ratio [OR] 1.26, 95% confidence interval [CI] 0.84– 1.88) compared to CMF. There was also no difference in the period prevalence at 6–7 years of age for pHF-W (OR 1.08, 95% CI 0.69–1.68) compared to CMF [17].
In addition, a more recent meta-analysis of 37 eligible intervention trials of hydrolyzed formula by Boyle et al. [18] reexamined the literature and was also less enthusiastic about the preventive effects of hydrolyzed formulas for eczema. The analysis suggested that there was “evidence of conflict of interest and high or unclear risk of bias in most studies of allergic outcomes and evidence of publication bias for studies of eczema and wheeze.” This analysis found no consistent evidence that pHF or eHF reduce the risk of allergic disease for infants at high risk [18]. In addition to more data, this analysis differs from others, as the results for different protein hydrolysates based on the degree of hydrolysis were pooled together. This may be inappropriate because the different biological effects of various hydrolysates are not only based on the degree of hydrolysis and peptide size, but also the qualitative characteristics of the peptide [8]. Additional differences include the interpretation of potential conflicts of interest from the studies included. Boyle et al. [18] also included studies in which in the intervention group, but not in the control group, additional interventions were applied such as house dust mite control measures and a smoke-free environment. Furthermore, studies carried out in a high-risk population and in the general population were pooled.
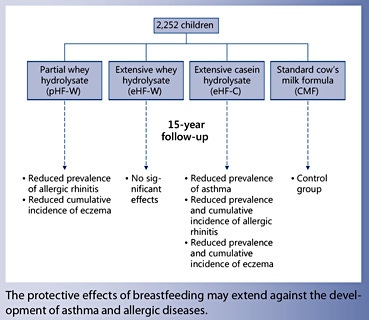
On the other hand, the most recent update of the German Infant Nutritional Intervention (GINI) study suggests a positive benefit from hydrolyzed formula for eczema prevention. The GINI study is a double-blind randomized trial to assess the effectiveness of 3 different types of hydrolyzed formulas: pHF-W and extensively hydrolyzed whey formula (eHF-W), an extensively hydrolyzed casein formula (eHF-C), and regular CMF on the development of allergic disease for children at high risk of developing allergy [19].
From 1995 to 1998, a total of 2,252 infants were enrolled and randomized at birth to receive 1 of the 4 formulas as a supplement to breastfeeding, as needed, during the first 4 months of life. The results from the GINI study suggest that the cumulative incidence of eczema up to 15 years of age was reduced in the pHF-W group (risk ratio [RR] 0.75, 95% CI 0.59– 0.96) and the eHF-C group (RR 0.60, 95% CI 0.46–0.77) compared to the CMF group. In addition, eczema prevalence between 11 and 15 years in the eHF-C group was decreased (OR 0.42, 95% CI 0.23–0.79) compared to the CMF group [20]. Cumulative incidence versus prevalence measures slightly different outcomes. Both outcomes are dichotomous; however, cumulative incidence includes anyone with a past or current diagnosis of eczema, while prevalence between 11 and 15 years indicates active disease during that specific time period. It is possible for a child to have developed the disease; however, it may become quiescent during a specific observation period. Further analysis suggests that based on the results of the GINI study, cost-effectiveness analyses suggest that both pHF-W and eHF-C can be cost-effective and cost-saving for the prevention of eczema [21].
Based on available evidence, in 2015, the US Food and Drug Administration (FDA) allowed a qualified health claim for pHF-W. Specifically, “for healthy infants who are not exclusively breastfed and who have a family history of allergy, feeding a 100% Whey-Protein Partially Hydrolyzed infant formula from birth up to 4 months of age instead of a formula containing intact cow’s milk proteins may reduce the risk of developing atopic dermatitis throughout the 1st year of life and up to 3 years of age. [The] FDA has concluded that the relationship between 100% Whey-Protein Partially Hydrolyzed infant formulas and the reduced risk of atopic dermatitis is uncertain, because there is very little scientific evidence for the relationship” [22, 23].
Hydrolyzed Formulas and Primary Food Allergy Prevention
The estimated prevalence of food allergy in the United States ranges from 2 to 10% [24], and in Europe, food allergy prevalence is estimated at approximately 6%, based on self-report [25]. One of the most common food allergies in children, cow’s milk protein allergy, peaks in infancy with an estimated prevalence of 2–6% [26]. Although hydrolyzed formulas are commonly used for treatment and management, there are many studies that have examined the use of hydrolyzed formulas for preventing the development of food allergies.
Using a randomized controlled trial design, Halken et al. [27] enrolled 595 high-risk Danish infants to compare the allergy-preventive effect of 3 different types of hydrolyzed formulas: eHF-C, eHF-W, or pHF-W during the first 4 months of life, as needed. All infants were followed up prospectively and if food allergy was suspected, controlled elimination/challenge procedures were performed. There were no differences in the cumulative incidence of atopic dermatitis or respiratory symptoms. Infants receiving pHF-W were found to be more likely to develop cow’s milk allergy (0.6 vs. 4.7%, p = 0.05); however, the authors cautioned that “because of the small number of cases the results should be interpreted with caution” [27]. Oldaeus et al. [28] assessed the effectiveness of eHF-C, pHF, or CMF in 155 high-risk infants for the development of allergic disease. Throughout the 18-month period, the infants in the eHF-C group did better than those in the CMF group, and for the first 9 months of age, the eHF group did better than the pHF group in terms of atopic symptoms [28].
These findings were also summarized in a 2009 Cochrane Review. There is some potential benefit for eHF versus pHF for food allergy prevention, based on 2 studies and 341 infants (typical RR 0.43, 95% CI 0.19–0.99). However, overall, there was limited evidence that prolonged feeding with a hydrolyzed formula compared to CMF reduces infant and childhood food allergy, food intolerance, or infant cow’s milk protein allergy for highrisk infants [29] . Since this review, there have been additional studies which seem to support these observations.
Kuo et al. [30] investigated whether feeding pHF-W versus CMF (any nonhydrolyzed protein formula) in the first 6 months of life to 1,002 high-risk infants decreased allergic diseases up to 36 months later. The percentage of infants with food sensitization, especially to milk protein, was significantly lower for infants in the pHF-W group compared to infants in the CMF group at 36 months (12.7 vs. 23.4%, p = 0.048); however, there was no difference in the prevalence of allergic diseases during the first 3 years of life [30]. Likewise, in the GINI study, no effect on food allergies was noted for infants randomized to hydrolyzed formulas. At 11–15 years of age, there were no differences in food sensitization for the children randomized to any of the hydrolyzed formulas, including pHF-W (OR 1.07, 95% CI 0.61–1.90), eHF-W (OR 1.10, 95% CI 0.63–1.94), or eHF-C (OR 1.20, 95% CI 0.69–2.10), when compared to CMF [20].
Hydrolyzed Formulas and Primary Asthma and Allergic Rhinitis Prevention
Both asthma and allergic rhinitis are common pediatric conditions. Asthma affects approximately 1 in 12 children in the United States [31] . It is associated with increased hospitalizations and emergency department visits, as well as racial and ethnic disparities in outcomes [32] . Globally, asthma is one of the most common, noncommunicable diseases in children [33] . Allergic rhinitis, also known as “hay fever” or allergic rhinoconjunctivitis, is a chronic condition characterized by conjunctivitis, rhinorrhea, nasal congestion, and pruritus. Allergic rhinitis affects 1 in 11 children [34] , and although the condition is not associated with frequent emergency department visits or hospitalizations, there are tremendous effects on quality of life, quality of nighttime sleep, and the ability to function at school [35] . The development of allergic rhinitis and asthma has been closely associated with the presence of eczema [36] . Similar to eczema, several studies have explored the effect of hydrolyzed formula in decreasing the likelihood of asthma and allergic rhinitis in children.
The development of allergic rhinitis and asthma has been closely associated with the presence of eczema
In the GINI study, although there were no effects on the development of asthma at 10 years of age [37], between 11 and 15 years of age [20], the prevalence of asthma was lower in the eHF-C group than in the CMF group (OR 0.49, 95% CI 0.26–0.89). These results were confirmed by objective spirometric testing. In terms of allergic rhinitis, the GINI study reported that the cumulative incidence of allergic rhinitis was lower in the eHF-C group (RR 0.77, 95% CI 0.59–0.99) than in the CMF group. In addition, the allergic rhinitis prevalence was lower for those children who received pHF-W (OR 0.67, 95% CI 0.47–0.95) and eHF-C (OR 0.59, 95% CI 0.41– 0.84) than for those who received CMF [20].
Overall, these results suggest that for those children who are not breastfed, compared to CMF, the early use of specific types of hydrolyzed formulas (pHF-W and eHFC) may have preventive effects for asthma and allergic rhinitis in children. Of note, eHF-W was not associated with any preventive effect. In addition, these findings from the GINI study are limited to children who are at high risk of allergic disease.
Hydrolyzed formulas have also been used in multipronged interventions. The Isle of Wight prevention study included a variety of interventions, including the use of hydrolyzed formula when breastfeeding was not possible. Starting in 1990, a total of 120 children at high risk of allergic disorders (based on family history and a high cord total IgE), were enrolled in a single-blinded, randomized controlled trial. Infants in the intervention arm were either breastfed with the mother placed on a low-allergen diet or, if not breastfed, they were fed a soybased protein hydrolysate formula. In addition, exposure to house dust mite allergen was reduced using vinyl mattress covers and acaricide in bedrooms and living rooms. The infants in the control group received routine care and no environmental control was recommended [38] . At the age of 18 years, 114 of 120 (95%) children were assessed and the prevalence of asthma was significantly lower in the prevention group compared with the control group (OR 0.34, 95% CI 0.12–0.96) [39].
The Canadian Childhood Asthma Primary Prevention Study also assessed a multifaceted intervention program for the primary prevention of asthma in high-risk infants. 545 high-risk infants were randomized to an intervention that included avoidance of house dust, pets, tobacco smoke, and encouragement of breastfeeding with delayed introduction of solid foods. pHF-W was provided for the first year of life as needed. At 7 years of age, the prevalence of asthma was lower in the intervention group (adjusted relative risk [ARR] 0.44, 95% CI 0.25–0.79); however, there were no differences in the prevalence of allergic rhinitis (ARR 1.13, 95% CI 0.71–1.81) and atopic dermatitis (ARR 0.92, 95% CI 0.49–1.73) [40].
Summary
Asthma, eczema, food allergy, and allergic rhinitis are some of the most common pediatric, chronic conditions in the world. Although breastfeeding is still regarded as the best approach to reduce the risk of allergy, for those infants who are exposed to infant formula, some studies suggest that certain pHF-W and eHF-C may decrease the risk of eczema compared to nonhydrolyzed formulas for children with a strong family history of atopic disease. However, the clinical interpretation of such studies varies, as do the subsequent clinical recommendations. Different professional medical societies have guidelines with varying levels of enthusiasm regarding the effectiveness of hydrolyzed formulas in preventing allergic disease, as well as which types of formulas are most effective [41– 43, 45].
In terms of allergic rhinitis, food allergy, and asthma, the current evidence for a preventive effect of hydrolyzed infant formula on these conditions seems to be inconsistent and insufficient. Finally, the qualitative changes to the peptides by the method of hydrolysis, not just the degree of protein hydrolysis, may have a large influence on the preventive effect of a particular infant formula for the potential risk of allergic disease. As a result, it may be difficult to generalize findings from clinical studies using a specific infant formula to other infant formulas from different manufacturers using different methods of hydrolysis. Further clinical studies are needed to help clinicians identify which infants may benefit from early intervention, as well as which specific hydrolyzed formulas are best suited to decrease the risk of future allergic disease.
Disclosure Statement
M.D.C. has served as a paid consultant for Nestlé, Abbott, Mead Johnson, and Wyeth. The writing of this article was supported by Nestlé Nutrition Institute.
References
- Federal Food, Drug and Cosmetic Act, 412, Title 21, Code of Federal Regulations 106.3 Definitions. http://www.accessdata.fda. gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch. cfm?fr=106.3 (accessed January 3, 2017).
- Centers for Disease Control and Prevention. Breastfeeding Report Card. https://www. cdc.gov/breastfeeding/data/reportcard.htm (accessed January 4, 2017).
- Black RE, Victoria CG, Walker SP, Bhutta ZA, Christian P, de Onis M, Ezzati M, Grantham-McGregor S, Katz J, Martorella R, Uauy R; Maternal and Child Nutrition Study Group: Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 2013; 382: 427–451.
- Corkins KG, Shurley T: What’s in the bottle? a review of infant formulas. Nutr Clin Pract 2016; 31: 723–729.
- Nutten S: Proteins, peptides and amino acids: role in infant nutrition. Nestle Nutr Inst Workshop Ser 2016; 86: 1–10.
- Ruth MR, Field CJ: The immune modifying effects of amino acids on gut-associated lymphoid tissue. J Anim Sci Biotechnol 2013; 4: 27.
- Strobel S: Dietary manipulation and induction of tolerance. J Pediatr 1992; 121:S74–S79.
- Lambers TT, Gloerich J, van Hoffen E, Alkema W, Hondmann DH, van Tol EAF: Clustering analyses in peptidomics revealed that peptide profiles of infant formulae are descriptive. Food Sci Nutr 2015; 3: 81–90.
- Ballard O, Morrow AL: Human milk composition: nutrients and bioactive factors. Pediatr Clin North Am 2013; 60: 49–74.
- Burke W, Fesinmeyer M, Reed K, Hampson L, Carlsten C: Family history as a predictor of asthma risk. Am J Prev Med 2003; 24: 160.
- Moore MM, Rifas-Shiman SL, Rich-Edwards JW, et al: Perinatal predictors of atopic dermatitis occurring in the first six months of life. Pediatrics 2004; 113: 468–474.
- United States Department of Health and Human Services and National Institute of Arthritis and Musculoskeletal and Skin Disease: Atopic Dermatitis. NIH Publication No. 03-4272. National Institutes of Health, 2003.
- Eichenfield LF, Hanifin JM, Luger TA, Stevens SR, Pride HB: Consensus conference on pediatric atopic dermatitis. J Am Acad Dermatol 2003; 49: 1088–1095.
- Gdalevich M, Mimouni D, David M, Mimouni M: Breast-feeding and the onset of atopic dermatitis in childhood: a systematic review and meta-analysis of prospective studies. J Am Acad Dermatol 2001; 45: 520– 527.
- Szajewska H, Horvath A: A meta-analysis of the evidence for a partially hydrolyzed 100% whey formula for the prevention of allergic diseases. Curr Med Res Opin 2010; 6: 423– 423.
- Alexander DD, Cabana MD: Partially hydrolyzed 100% whey protein infant formula and reduced risk of atopic dermatitis: a metaanalysis. J Pediatr Gastroenterol Nutr 2010; 50: 356–358.
- Lowe AJ, Hosking CS, Bennett CM, Allen KJ, Axelrad C, Carlin JB, Abramson MJ, Dharmage SC, Hill DJ: Effect of a partially hydrolyzed whey infant formula at weaning on risk of allergic disease in high-risk children: a randomized controlled trial. J Allergy Clin Immunol 2011; 128: 360–365.
- Boyle RJ, Ierodiakonou D, Khan T, Chivinge J, Robinson Z, Geoghegan N, Jarrold K, Afxentiou T, Reeves T, Cunha S, Trivella M, Garcia-Larsen V, Leonardi-Bee J: Hydrolysed formula and risk of allergic or autoimmune disease: systematic review and metaanalysis. BMJ 2016; 352:i974.
- von Berg A, Koletzko S, Grubl A, Filipiak- Pittroff B, Wichmann HE, Bauer CP, Reinhardt D, Berdel D: The effect of hydrolyzed cow’s milk formula for allergy prevention in the first year of life: the German Infant Nutritional Intervention Study, a randomized double-blind trial. J Allergy Clin Immunol 2003; 111: 533–540.
- von Berg A, Filipiak-Pittroff B, Schulz H, Hoffmann U, Link E, Sußmann M, Schnappinger M, Brüske I, Standl M, Krämer U, Hoffmann B, Heinrich J, Bauer CP, Koletzko S, Berdel D; GINIplus study group: Allergic manifestation 15 years after early intervention with hydrolyzed formulas – the GINI Study. Allergy 2016; 71: 210–219.
- Mertens J, Stock S, Lüngen M, von Berg A, Krämer U, Filipiak-Pittroff B, Heinrich J, Koletzko S, Grübl A, Wichmann HE, Bauer CP, Reinhardt D, Berdel D, Gerber A: Is prevention of atopic eczema with hydrolyzed formulas cost effective? A health economic evaluation from Germany. Pediatr Allergy Immunol 2012; 23: 597–604.
- United States Food and Drug Administration: 100% Whey-Protein Partially Hydrolyzed Infant Formula and Reduced Risk of Atopic Dermatitis. http://www.fda.gov/ Food/IngredientsPackagingLabeling/LabelingNutrition/ ucm256731.htm (accessed February 4, 2017).
- Chung CS, Yamini S, Trumbo PR: FDA’s health claim review: whey-protein partially hydrolyzed infant formula and atopic dermatitis. Pediatrics 2012; 130:e408–e414.
- Sicherer SH, Sampson HA: Food allergy: epidemiology, pathogenesis, diagnosis, and treatment. J Allergy Clin Immunol 2014; 133: 291–307.
- Nwaru BI, Hickstein L, Panesar SS, et al: The epidemiology of food allergy in Europe: a systematic review and meta-analysis. Allergy 2014; 69: 62–75.
- Host A: Frequency of cow’s milk allergy in childhood. Ann Allergy Asthma Immunol 2002; 89:s33–s37.
- Halken S, Hansen KS, Jacobsen HP, Estmann A, Faelling AE, Hansen LG, Kier SR, Lassen K, Lintrup M, Mortensen S, Ibsen KK, Osterballe O, Høst A: Comparison of a partially hydrolyzed infant formula with two extensively hydrolyzed formulas for allergy prevention: a prospective, randomized study. Pediatr Allergy Immunol 2000; 11: 149–161.
- Oldaeus G, Anjou K, Bjorksten B, Moran JR, Kjellman NI: Extensively and partially hydrolysed infant formulas for allergy prophylaxis. Arch Dis Child 1997; 77: 4–10.
- Osborn DA, Sinn JKH: Formulas containing hydrolysed protein for prevention of allergy and food intolerance in infants. Cochrane Database Syst Rev 2006;CD003664.
- Kuo HC, Liu CA, Ou CY, Hsu TY, Wang CL, Huang HC, Chuang H, Liang HM, Yang KD: Partial protein-hydrolyzed infant formula decreased food sensitization but not allergic disease in a prospective birth cohort study. Int Arch Allergy Immunol 2011; 154: 310– 317.
- Akinbami LJ, Simon AE, Rossen LM: Changing trends in asthma prevalence among children. Pediatrics 2016; 137: 1.
- Cabana MD, Lara M, Shannon J: Racial and ethnic disparities in the quality of asthma care. Chest 2007; 132(suppl 5):810S–817S.
- Zar HJ, Ferkol TW: The global burden of respiratory disease-impact on child health. Pediatr Pulmonol 2014; 49: 430–434.
- Bloom B, Cohen RA, Freeman G: Summary health statistics for US children: national health interview survey, 2011. Vital Health Stat 2012; 10: 1–88.
- Blaiss MS: Allergic rhinoconjunctivitis: burden of disease. Allergy Asthma Proc 2007; 28: 393–397.
- Dharmage SC, Lowe AJ, Matheson MC, Burgess JA, Allen KJ, Abramson MJ: Atopic dermatitis and the atopic march revisited. Allergy 2014; 69: 17–27.
- von Berg A, Filipiak-Pittroff B, Krämer U, Hoffmann B, Link E, Beckmann C, Hoffmann U, Reinhardt D, Grübl A, Heinrich J, Wichmann HE, Bauer CP, Koletzko S, Berdel D: Allergies in high-risk school children after early intervention with cow’s milk protein hydrolysates: 10-year results from the German Infant Nutritional Intervention (GINI) study. J Allergy Clin Immunol 2013; 131: 1565–1573.
- Hide DW, Matthews S, Matthews L, Stevens M, Ridout S, Twiselton R, Gant C, Arshad SH: Effect of allergen avoidance in infancy on allergic manifestations at age two years. J Allergy Clin Immunol 1994; 93: 842–846.
- Scott M, Roberts G, Kurukulaaratchy RJ, Matthews S, Nove A, Arshad SH: Multifaceted allergen avoidance during infancy reduces asthma during childhood with the effect persisting until age 18 years. Thorax 2012; 67: 1046–1051.
- Chan-Yeung M, Ferguson A, Watson W, Dimich-Ward H, Rousseau R, Lilley M, Dy- Buncio A, Becker A: The Canadian Childhood Asthma Primary Prevention Study: outcomes at 7 years of age. J Allergy Clin Immunol 2005; 116: 49–55.
- Australasian Society of Clinical Immunology and Allergy (ASCIA) 2016 Guidelines – infant feeding and allergy prevention. https://www.allergy.org.au/health-professionals/ papers/ascia-guidelines-for-infantfeeding- and-allergy-prevention (accessed January 21, 2017).
- Chan ES, Cummings C; Canadian Paediatric Society, Community Paediatrics Committee and Allergy Section: Dietary exposures and allergy prevention in high-risk infants: a joint statement with the Canadian Society of Allergy and Clinical Immunology. Paediatr Child Health 2013; 18: 545–549.
- Sampson HA, Aceves S, Bock SA, James J, Jones S, Lang D, Nadeau K, Nowak-Wegrzyn A, Oppenheimer J, Perry TT, Randolph C, Sicherer SH, Simon RA, Vickery BP, Wood R; Joint Task Force on Practice Parameters, et al: Food allergy: a practice parameter update, 2014. J Allergy Clin Immunol 2014; 134: 1016–1025.
- Tsabouri S, Douros K, Priftis KN: Cow’s milk allergenicity. Endocr Metab Immune Disord Drug Targ 2014; 14: 16–26.
- di Mauro G, Bernardini R, Barberi S, et al: Prevention of food and airway allergy: consensus of the Italian Society of Preventive and Social Paediatrics, the Italian Society of Paediatric Allergy and Immunology, and Italian Society of Pediatrics. World Allergy Organ J 2016; 9: 28.