Physiological Effects of Feeding Infants and Young Children Formula Supplemented with Milk Fat Globule Membranes
Abstract
Dietary supplementation with bovine milk fat globule membrane (MFGM) concentrates has recently emerged as a possible means to improve the health of infants and young children. Formula-fed infants are of special interest since infant formulas traditionally have lower concentrations of biologically active MFGM components than human milk. We iden- tified 6 double-blind randomized controlled trials (DBRCT) exploring the effects of supple- menting the diet of infants and children with bovine MFGM concentrates. Two studies found a positive effect on cognitive development in formula-fed infants. Three studies found a protective effect against infections at different ages during infancy and early child- hood. We conclude that supplementation with MFGM during infancy and childhood appears safe, and the studies indicate positive effects on both neurodevelopment and defense against infections, especially in formula-fed infants. However, due to the small number of studies and the heterogeneity of interventions and outcomes, more high-quality DBRCTs are needed before firm conclusions can be drawn on the likely health benefits of MFGM supplementation to infants and children.
Introduction
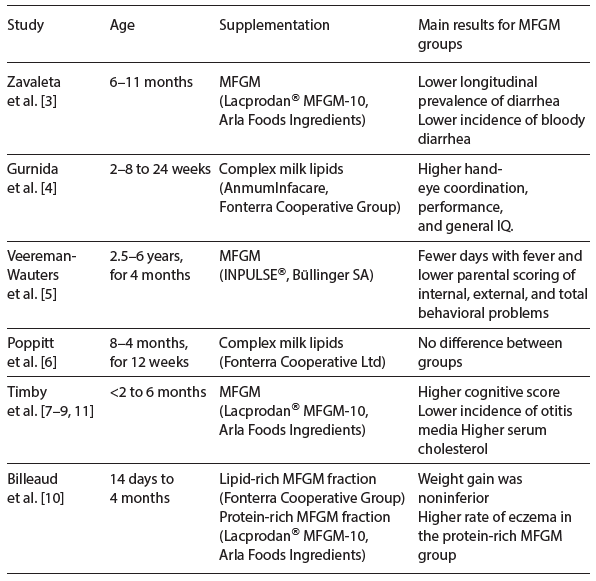
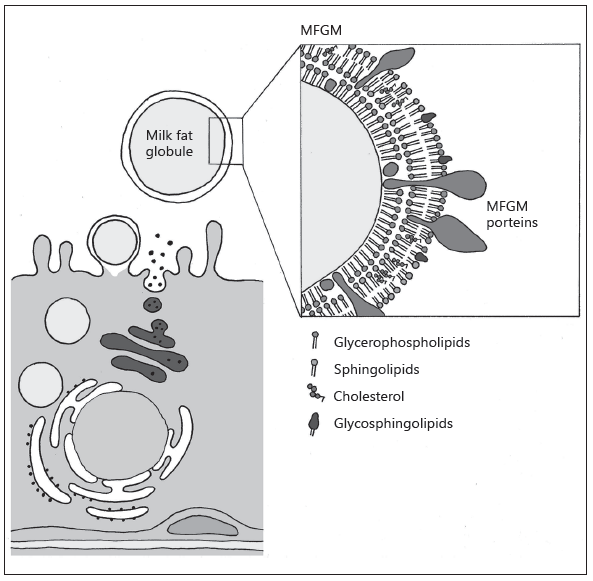
An increasing number of studies have reported various health benefits from oral supplementation with bovine milk fat globule membrane (MFGM) to humans of different ages, including infants and children [1, 2]. The MFGM is formed during the release of milk fat from the endothelial cell of the lactating mammary gland and is composed of a phospholipid and cholesterol triple layer which contains proteins and glycoproteins [3] (Fig. 1). Milk phospholipids, sphingomyelins, and gangliosides are largely located on the MFGM, although phospholipids are also secreted as smaller vesicles devoid of a triglyceride core, which typically separate from the whey fraction [3, 4]. The proteome of the human MFGM is very complex with several hundred proteins identified, including mucins, butyrophilin, lactoferrin, and lactadherin [5, 6]. Bovine MFGM-rich fractions contain approx- imately the same number of proteins [7]. MFGM is also rich in sialic acid as part of gangliosides [4] and glycosylated proteins. The genes regulating MFGM syn- thesis are conserved across species suggesting a functional benefit of this fraction in milk [8], even if the detailed MFGM composition varies among species [6].
Breastfed infants have a higher intake of MFGM components than formula- fed infants because, traditionally, the MFGM fraction is discarded with the milk fat which is replaced by blends of vegetable oils as the source of fat in infant formulas. Resulting from advances in dairy technology, bovine MFGM concentrates are now commercially available and possible to use as a supplement to foods, including infant formulas.
Physiological Effects of Single Components of the Milk Fat Globule Membrane
Dietary gangliosides [9], sialic acid [10], and sphingomyelin [11] have been shown to be important for optimal brain development and function in different animal models. However, it should be noted that some of these models are disease models or models with inhibited de novo synthesis, which is far from supplementing a healthy infant or child. In a small study on premature infants with a birth weight <1,500 g, infants receiving formula with high sphingomyelin content (20 vs. 13% of all phospholipids in milk) to cover shortages of breast milk performed better than those fed the lower content at neurobehavioral follow-up between 6 and 18 months corrected age [12]. Further, oral sphingomyelin [13], as well as a bovine MFGM concentrate [14], increased maturation of the intestine in rats. Gangliosides have also been suggested to play an important role in the development of intestinal microbiota composition, gut immunity, and, consequently, in the defense against infections [15]. Other components of MFGM are also involved in the defense against infections, e.g., the glycoproteins butyrophilin, lactadherin, and mucins [16], which all have antimicrobial effects, and the lipid fraction of bovine MFGM has antiviral effects in vitro [17]. Both lipid and protein components of MFGM have anticancer effects in vitro [18], and intake of MFGM in early life has also been suggested to protect against obesity later in life [19].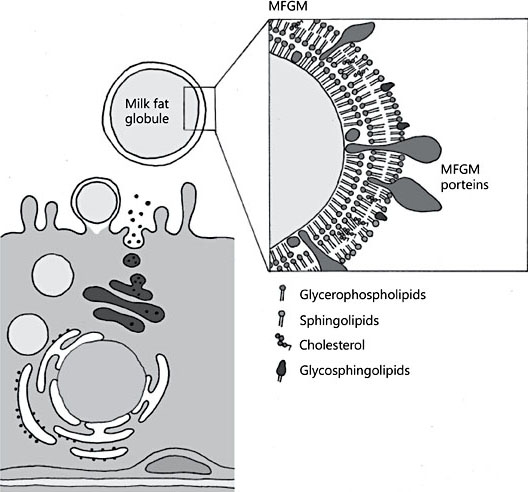
Illustration by Erik Domellöf. Reproduced from Hernell et al. [1] with permission.
Clinical Studies on Milk Fat Globule Membrane Concentrates Fed to Infants and Children
In a literature search (August 31, 2017), we identified 6 double-blind randomized controlled trials (DBRCT) exploring the effects of supplementing the diet of infants or children with MFGM (Table 1):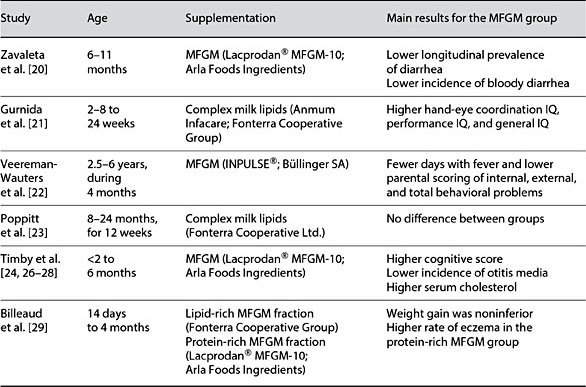
In a Peruvian DBRCT, 550 healthy, primarily breastfed 6- to 11-month-old infants consumed 40 g/day of an instant complementary food fortified with 1 recommended dietary allowance of multiple micronutrients and a protein source for 6 months. They were randomized to the protein source being either an MFGM-enriched protein fraction (Lacprodan® MFGM-10; Arla Foods Ingredients, Viby, Denmark) or skim milk powder (control group) [20]. There was no difference between the groups in the incidence of diarrhea, but longitudinal prevalence of diarrhea was significantly lower in the MFGM group compared to the control group (3.84 vs. 4.37%, p < 0.05). In a multivariate model adjusted for initial anemia and potable water facilities, the incidence of bloody diarrhea was lower in the MFGM group, with an adjusted OR of 0.59 (95% CI 0.34–1.02, p = 0.025).
In a DBRCT performed in Indonesia, 70 term infants were randomized to a control formula or an infant formula enriched with bovine milk gangliosides, provided as a complex bovine milk lipid fraction (AnmumInfacare; Fonterra Cooperative Group, Auckland, New Zealand) [21]. A breastfed reference group (BFR) (n = 40) was also recruited. The intervention started between 2 and 8 weeks and continued until 24 weeks of age. After adjustment for socioeconomic background variables, the hand-eye coordination IQ (129.5 vs. 122.0, p =0.006), performance IQ (131.1 vs. 123.2, p < 0.001), and general IQ (125.4 vs.120.6, p = 0.041) measured with the Griffiths Mental Developmental Scale were higher in the ganglioside-supplemented group than in the control group, and the ganglioside-supplemented group did not differ from the BFR group.
In a Belgian DBRCT, 253 preschool children aged 2.5–6 years received 200 mL of a chocolate formula milk daily for 4 months [22]. They were randomized to a formula without phospholipids (placebo group) or enriched with 500 mg of phospholipids with the addition of 2.5% of a phospholipid-rich MFGM concentrate (Inpulse; Büllinger SA, Büllingen, Belgium) (intervention group). The intervention group had fewer days with fever (mean ± SD: 1.71 ± 2.47 vs.2.60 ± 3.06, p = 0.028), and lower parental scoring of internal (p < 0.003), external (p < 0.005), and total (p < 0.002) behavioral problems measured by the Achenbach System of Empirically Based Assessment (ASEBA). However, ASEBA scoring was only performed after the intervention but not at baseline, and differences were not confirmed when the children’s teachers made the scoring.
In an Indian DBRCT, 450 infants between 8 and 24 months of age were randomized to a daily dose of milk powder supplemented with 2 g of a spray-dried ganglioside concentrate (Fonterra Cooperative Ltd,) or milk powder only (control group) for 12 weeks [23]. There was no difference between the groups, nor in the primary outcome rotavirus diarrhea, or in secondary outcomes including all-cause diarrhea. However, the authors noted that the incidence of rotavirus diarrhea during the study period was lower than expected, making the study under-powered as compared to the intention of the design.
In a Swedish DBRCT, 160 formula-fed healthy term infants were randomized to receive an experimental formula (EF) supplemented with a protein-rich MFGM fraction (Lacprodan® MFGM-10; Arla Foods Ingredients) or standard formula (SF) from <2 to 6 months of age. The EF had lower energy (60 vs. 66 kcal/100 mL) and protein (1.20 vs. 1.27 g/100 mL) densities, and MFGM-proteins made up 4% (wt/wt) of the total protein content in the formula. In addition, a BFR group including 80 infants was also studied. The formula-fed infants regulated their ingested volumes by increasing meal size, resulting in no differences in energy intake, protein intake, blood urea nitro- gen (BUN), serum insulin level, or growth, including body fat percentage, up to 12 months of age [24]. The surprisingly high level of self-regulation for the bottle-fed infants might be explained by a low level of parental control in the study population [25].
At 12 months of age, the EF group achieved higher scores (mean ± SD) in the cognitive domain of Bayley III (105.8 ± 9.2) than the SF group (101.8 ± 8.0, p = 0.008) and did not differ from the BFR group (106.4 ± 9.5, p = 0.73) [24]. During the intervention, the EF group had a lower incidence of acute otitis media than the SF group (1 vs. 9%, p = 0.034), a lower incidence and longitudinal prevalence of antipyretic use, lower concentrations of serum IgG against pneumococci after vaccination and a lower prevalence of Moraxella catarrhalis in the oral microbiota, all suggesting an infection-protective effect of EF [26, 27]. During the intervention, the EF group gradually reached higher serum cholesterol concentrations than the SF group, and there was no significant difference between the EF and BFR group at 6 months of age [28].
In a multicenter noninferiority DBRCT, 199 healthy term infants were randomized to 3 different formulas from 14 days to 4 months of age; a SF (control), a formula enriched with lipids (MFGM-L; Fonterra Cooperative Group Ltd), and a formula with a protein-rich (MFGM-P, Lacprodan® MFGM-10, Arla Foods Ingredients) bovine MFGM fraction, respectively [29]. Weight gain was noninferior in the MFGM-L and MFGM-P groups compared with the control group. Adverse events and morbidity rates were similar across groups except for a higher rate of eczema in the MFGM-P group (13.9 vs. 1.4% in the MFGM-L group and 3.5% in the control group, p = 0001). It is, however, not clear how and when eczema was diagnosed, and the number of infants diagnosed were few in the MFGM-L and control groups (1 and 2, respectively). The authors also concluded that care must be taken in interpreting the exploratory endpoints. A higher risk of skin rash was not confirmed in a Swedish study [30] which studied the same MFGM-P fraction.
Conclusions
Studies on the supplementation of bovine MFGM to the diet of infants and children have shown promising results regarding both neurodevelopment and defense against infections. These findings are supported by known effects of individual components of MFGM mostly based on in vitro and/or animal studies. However, the scientific base of knowledge for MFGM supple- mentation to infants and children is still limited. The number of published studies on MFGM supplementation to infants and children is small, and the interventions are heterogeneous: different MFGM concentrates have been given for different durations at different ages and with different main out- comes. However, MFGM supplementation seems safe down to the age of the first week of life in term infants, as no serious adverse effects have been reported.
Infant formulas supplemented with bovine MFGM concentrates have already been launched on many markets, but before any general recommendations or guidelines of MFGM use in infants and children can be given, more high-quality DBRCTs are needed.
References
- 1 Hernell O, Timby N, Domellöf M, Lönnerdal B: Clinical benefits of milk fat globule membranes for infants and children. J Pediatr 2016;173(suppl):S60–S65.
- 2 Timby N, Domellöf M, Lönnerdal B, Hernell O: Supplementation of infant formula with bovine milk fat globule membranes. Adv Nutr 2017;8:351–355.
- 3 Bourlieu C, Michalski MC: Structure-function relationship of the milk fat globule. Curr Opin Clin Nutr Metab Care 2015;18:118–127.
- 4 Rueda R, Maldonado J, Narbona E, Gil A: Neonatal dietary gangliosides. Early Hum Dev 1998;53(suppl):S135–S147.
- 5 Liao Y, Alvarado R, Phinney B, Lönnerdal B: Proteomic characterization of human milk fat globule membrane proteins during a 12 month lactation period. J Proteome Res 2011; 10:3530–3541.
- 6 Lu J, Wang X, Zhang W, et al: Comparative proteomics of milk fat globule membrane in different species reveals variations in lactation and nutrition. Food Chem 2016;196: 665–672.
- 7 Affolter M, Grass L, Vanrobaeys F, et al: Qualitative and quantitative profiling of the bovine milk fat globule membrane proteome. JProteomics 2010;73:1079–1088.
- 8 German JB: Dietary lipids from an evolution- ary perspective: sources, structures and func- tions. Matern Child Nutr 2011;7(suppl 2):2– 16.
- 9 Palmano K, Rowan A, Guillermo R, et al: The role of gangliosides in neurodevelopment. Nutrients 2015;7:3891–3913.
- 10 Wang B: Sialic acid is an essential nutrient for brain development and cognition. Annu Rev Nutr 2009;29:177–222.
- 11 Oshida K, Shimizu T, Takase M, et al: Effects of dietary sphingomyelin on central nervous system myelination in developing rats. PediatrRes 2003;53:589–593.
- 12 Tanaka K, Hosozawa M, Kudo N, et al: The pilot study: sphingomyelin-fortified milk has a positive association with the neurobehavioural development of very low birth weight infants during infancy, randomized control trial. Brain Dev 2013;35:45–52.
- 13 Motouri M, Matsuyama H, Yamamura J, et al: Milk sphingomyelin accelerates enzymatic and morphological maturation of the intestine in artificially reared rats. J Pediatr Gas- troenterol Nutr 2003;36:241–247.
- 14 Bhinder G, Allaire JM, Garcia C, et al: Milk fat globule membrane supplementation in formula modulates the neonatal gut microbiome and normalizes intestinal development. Sci Rep 2017;7:45274.
- 15 Rueda R: The role of dietary gangliosides on immunity and the prevention of infection. Br J Nutr 2007;98(suppl 1):S68–S73.
- 16 Liu B, Newburg DS: Human milk glycopro- teins protect infants against human pathogens. Breastfeed Med 2013;8:354–362.
- 17 Fuller KL, Kuhlenschmidt TB, Kuhlen- schmidt MS, et al: Milk fat globule membrane isolated from buttermilk or whey cream and their lipid components inhibit infectivity of rotavirus in vitro. J Dairy Sci 2013;96:3488– 3497.
- 18 Spitsberg VL: Invited review: bovine milk fat globule membrane as a potential nutraceuti- cal. J Dairy Sci 2005;88:2289–2294.
- 19 Baars A, Oosting A, Engels E, et al: Milk fat globule membrane coating of large lipid droplets in the diet of young mice prevents body fat accumulation in adulthood. Br J Nutr 2016;115:1930–1937.
- 20 Zavaleta N, Kvistgaard AS, Graverholt G, et al: Efficacy of an MFGM-enriched complementary food in diarrhea, anemia, and micronutrient status in infants. J Pediatr Gas- troenterol Nutr 2011;53:561–568.
- 21 Gurnida DA, Rowan AM, Idjradinata P, et al: Association of complex lipids containing gangliosides with cognitive development of 6-month-old infants. Early Hum Dev 2012; 88:595–601.
- 22 Veereman-Wauters G, Staelens S, Rombaut R, et al: Milk fat globule membrane (IN- PULSE) enriched formula milk decreases febrile episodes and may improve behavioral regulation in young children. Nutrition 2012; 28:749–752.
- 23 Poppitt SD, McGregor RA, Wiessing KR, et al: Bovine complex milk lipid containing gangliosides for prevention of rotavirus infection and diarrhoea in northern Indian infants. J Pediatr Gastroenterol Nutr 2014;59:167–171.
- 24 Timby N, Domellöf E, Hernell O, et al: Neuro- development, nutrition, and growth until 12 mo of age in infants fed a low-energy, low-protein formula supplemented with bovine milk fat globule membranes: a randomized controlled trial. Am J Clin Nutr 2014;99:860–868.
- 25 Timby N, Hernell O, Lönnerdal B, et al: Pa- rental feeding control in relation to feeding mode and growth pattern during early infancy. Acta Paediatr 2014;103:1072–1077.
- 26 Timby N, Hernell O, Vaarala O, et al: Infections in infants fed formula supplemented with bovine milk fat globule membranes. J Pediatr Gastroenterol Nutr 2015;60:384– 389.
- 27 Timby N, Domellöf M, Holgerson PL, et al: Oral microbiota in infants fed a formula sup- plemented with bovine milk fat globule membranes – a randomized controlled trial. PLoS One 2017;12:e0169831.
- 28 Timby N, Lönnerdal B, Hernell O, et al: Car- diovascular risk markers until 12 mo of age in infants fed a formula supplemented with bovine milk fat globule membranes. Pediatr Res 2014;76:394–400.
- 29 Billeaud C, Puccio G, Saliba E, et al: Safety and tolerance evaluation of milk fat globule membrane-enriched infant formulas: a randomized controlled multicenter non-inferiority trial in healthy term infants. Clin Med Insights Pediatr 2014;8:51–60.
- 30 Timby N, Domellöf M, Lönnerdal B, et al: Comment on “Safety and tolerance evaluation of milk fat globule membrane-enriched infant formulas: a randomized controlled multicenter non-inferiority trial in healthy term infants”. Clin Med Insights Pediatr 2015;9:63–64.