Nutrition for Preterm Infants: 75 Years of History
Key Messages
• Early nutrition has a huge impact on the short- and long-term health of preterm infants.
• Human milk has been and is again the preferred enteral supply, although fortification is required to meet the requirements.
Keywords
Iron · Folate · Human milk · Donor milk · Fortifier · Probiotics
Abstract
As technology has advanced, survival rates of preterm infants have improved dramatically. Human milk was the primary source of enteral nutrition during the early days of neonatology, but the HIV/AIDS epidemic resulted in an increased use of preterm formula. More recently, the benefits of human milk were rediscovered, resulting in increased use of donor human milk as well. The awareness that human milk does not contain the amounts of nutrients to meet the high requirements of infants born premature resulted in the development of human milk fortifiers. The development of these fortifiers is still ongoing, as are alternative methods of pasteurization of donor milk. Those initiatives will increase the use of human milk with consequently short- and long- term benefits for preterm infants.
Introduction
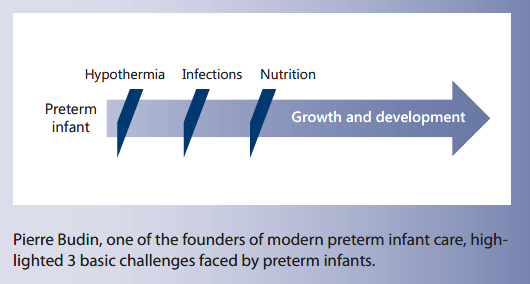
Modern preterm infant care has its origins at the Hôpital de la Charité in Paris. Pierre Budin, influenced by the former head midwife of the Maternité, Madame Henry, installed the first specialized care unit for “weaklings,” as underweight infants were then referred to, in 1893 (Fig. 1). Budin was then called as head of Obstectrics at Hôpital de la Maternité in 1898. Under his direction, both hospitals became the world’s first centers of specialized study on caring for preterm infants. In his lectures to students, published in 1890, Baudin highlighted 3 main basic problems for preterm infants:
• Risk of hypothermia through cooling
• Vulnerability to infections
• Feeding
Although the first neonatal unit was already opened in 1893, it took a long time before anything was published on preterm nutrition in the scientific literature and espe- cially in this journal.
The First Article on Preterm Nutrition in Annales Nestlé (Annals of Nutrition and Metabolism)
The first article that appeared in the Annals of Nutrition and Metabolism which was somehow related to nutrition and neonates was published by Zittoun et al. [1] from France in 1983. They investigated the effect of iron supplementation during pregnancy, and this placebo-controlled (!) trial demonstrated that iron supplements to iron-deprived mothers had no effect on the ferritin status of the newborn, on the folate status of the mothers or infants, or on the frequency of obstetrical complications. A significant relationship was found between maternal folate levels and length of gestation. The authors concluded that folate supplementation during pregnancy might reduce the incidence of premature delivery.
Folate and Iron: Present Knowledge and Advice
That folate was important became more obvious in the following years, but not so much with regard to anemia, but as an actor in prevention of neural tube defects. Unambiguous evidence of the effectiveness of periconceptional folic acid in preventing neural tube defects has been available since 1991 [2] and is still undisputed [3]. The first governments to formulate a periconceptional folic acid supplementation policy were the United Kingdom (1992), Ireland (1993), and the Netherlands (1993); six more (Switzerland 1996, Denmark 1997, Norway 1998, Portugal 1998, France 2000, and Spain 2001) followed. Despite firm evidence, adherence is low.
Many studies have appeared regarding maternal iron supplementation since 1983, and at present it is clear that iron supplementation reduces maternal anemia incidence in pregnancy but the effects on infant outcomes are less clear [4]. Compared with controls, women taking iron supplements less frequently had low-birth-weight newborns (8.4 vs. 10.3%, average RR 0.84, 95% CI 0.69– 1.3 , 11 trials, 17,613 women; low-quality evidence) and preterm babies (RR 0.93, 95% CI 0.84–1.03, 13 trials, 19,286 women; moderate-quality evidence).
Preterm and low-birth-weight infants are at high risk of iron deficiency due to low iron stores at birth and high- er iron requirements due to rapid growth. Already in 1971, Lundstrom et al. [5] demonstrated that 2 mg/kg was adequate to prevent anemia in infants with a birth weight of 1,000–2,000 g at 3 months of age. The randomization procedure was that infants with odd birth dates received 2 mg iron as ferrous sulfate/kg/day starting at 0.5 months; those with even birth dates received no additional iron unless they developed anemia. Interestingly, almost 50 years later, with many additional randomized controlled trials performed, the most recent recommendation (published in the Annals of Nutrition an Metabolism December 2017 issue) is almost identical: 1–3 mg/kg/day (1–2 mg for marginally low birth weight and 2–3 mg for very low birth weight) is needed to effectively prevent iron deficiency [6]. There is some recent evidence that these levels of iron intake will prevent some of the negative health consequences associated with low birth weight, especially behavioral problems and other neurodevelopmental outcomes and possibly even hypertension.

Fig. 1. “Maternité de Paris” from The Illustrated London News, March 8th, 1884.
Milestones in Enteral Preterm Nutrition
Preterm Formula
Since the early days of caring for preterm infants, it has been widely held that human milk was the food of choice for these infants. This belief, however, has not prevented some pediatricians from suggesting that human milk might not in fact be the ideal food on the grounds that its low protein content is insufficient for growth requirements. Especially the lack of adequate protein content to meet the requirements has driven the development of formulas especially designed for preterm infants. One of the earlier publications on protein content of preterm formula originated from Professor Peter Davies in 1975 [7]. He acknowledged that adequate protein intake in the early weeks of life is necessary if growth is to proceed normally. The question of optimum protein requirements for preterm infants is therefore an important one. He investigated 106 preterm infants who were fed 1 of 3 isocaloric milks for a period of 2 months. Milk A was high-protein milk (21% calories as protein), milk B was medium-protein milk (15% calories as protein), and milk C was human breast milk (7% calories as protein). Changes in weight, length, head circumference, and triceps skinfold thickness were evaluated. The results suggested that though human milk was adequate for the growth needs of the more mature preterm infants (33–36 weeks’ gestation), less mature infants (28–32 weeks’ gestation) fed human milk failed to achieve adequate growth rates compared with infants on higher protein intakes. At the same time, a landmark paper by Goldman et al. [8] appeared in the Journal of Pediatrics, warning about very high protein intake because of deleterious effects on IQ development. This study was a follow-up of an earlier double-blinded study, performed in 1963 [9], where less edema was observed in infants fed 6 g/kg/day of cow’s milk protein, but more fever, lethargy, and poor feeding behavior as well as higher levels of plasma protein than the infants fed 3 g/kg/day of cow’s milk protein in the direct postnatal phase. Specifically infants with a birth weight <1,300 g were at risk of developing strabismus and low IQ scores.
As product development enhanced, casein whey fractions were modified to increase tolerability, many studies addressed the optimal intake and relationship between energy and protein intakes.
Cow’s milk contains approximately 20% whey protein and 80% casein proteins, whereas formulas were gradually modified to a ratio of whey proteins to caseins of 60:40. These modifications resulted in a plasma amino acid pattern more resembling that of a fully breastfed infant [10]. Pivotal in that development were the studies by Kashyap and Heird [11, 12] who studied the effects of varying protein and energy intakes. These studies fol- lowed the observation that premature infants have high rates of energy expenditure, using different techniques [13, 14]. Acknowledging that sufficient energy must be provided for maintaining high rates of protein synthesis, many studies were undertaken to determine the optimal protein energy ratio in vari-ous groups [15]. This resulted in recommendations that enterally fed infants should receive 110–135 kcal/kg/day and at least 3.6 g/kg/day of proteins [16].
While the breast milk of mothers who deliver preterm contains higher amounts of proteins, this effect diminishes after approximately 1 month. The quality of the milk may also be different, especially with regard to the immune proteins [17, 18]. These observations led to a general believe that human milk did not meet the requirements of rapidly developing preterm infants with subsequent effects on growth and development. This led to the development of formulas specifically designed for pre-term infants. An important paper published in The Lancet in 1990 [19] suggested that infants fed a higher nutrient density formula for a period as short as 1 month had long lasting effects on neurodevelopment and regional brain volumes [19–21]. Preterm formula became standard feeding in many neonatal units, and rates of infants fed own mother’s milk declined significantly. Editorials with suggestive titles such as “Breast Not Necessarily Best” appeared in 1998, in an era in which AIDS and its effects became very apparent [22]. However, already 5 years later, arguments were made to use own mother’s milk because of evidence that human milk reduced rates of infections and necrotizing enterocolitis (NEC) and enhanced the developmental outcome of preterm infants [23].
Donor Milk
Approximately 75 years ago, human donor milk be- came increasingly popular throughout Europe. Especially after the Second World War, many countries started a human milk bank. Figure 2 shows the workflow in these days. Milk was collected at home, brought to a central place (blood bank or hospital) and vacuum dried. Powered human milk was available for preterm born infants and neonates with gastrointestinal problems.
With the discovery of HIV and knowing that breast milk could serve as transmitter, many milk banks closed throughout Europe. Concomitantly, preterm formula was developed. Although preterm formula administration results in higher in-hospital growth rates than own mother’s milk, the use of cow’s milk-based preterm formula when own mother’s milk was not available has not been without controversy. Already in 1990, Lucas and Cole [24] reported that in exclusively formula-fed babies, NEC was 6–10 times more common than in those fed breast milk alone and 3 times more common than in those who received formula plus breast milk. Numerous papers report also an association with a lower incidence of nosocomial infections when own mother’s milk is fed instead of preterm formula [e.g., 25]. Following these observations and implementing methods to prevent the transmission of contagious microbes in human milk, the use of donor milk as substitute for own mother’s milk be- came increasingly popular again. At present, more than 500 donor milk banks are operating around the world, most of them in Europe and South America. Processing techniques have been optimized and guidelines on the preferential use have been issued [26]. Preservation techniques have been optimized (Fig. 3), increasing the length of storage and therewith the price [27].
The exact mechanism as to how human milk exerts a beneficial effect on the prevalence of NEC is at present unknown. Many factors might contribute to the particularly increased risk of preterm infants to develop NEC (Table 1). The altered microbiome in addition to an altered immune response may be the two most important mechanisms. The colonization of the gut of the preterm infant is progressing slower than that of a term infant, while the numbers of microbiota are reduced and less diverse. That may be influenced by the high prevalence of antibiotic use in the first period of life [28], the environment (a NICU with many selected pathogens [29]), the altered mucin production rates and quality [30], the motility [31], and the immune system.
The fetal immune system is generally characterized as tolerogenic to prevent graft-versus-host responses during pregnancy. T cells in the fetal intestinal mucosa allow for early compartmentalization of TH1 responses, predominated by TNF-α and IL-2-producing T cells early in human development [32, 33]. These fetal mucosal TH1 cells can contribute to mucosal development by producing TNF-α, which promotes the outgrowth of fetal intestinal epithelial stem cells. However, when this process is disturbed by preterm birth, preferential induction of TH1 cells may instigate an inflammatory cascade providing the underlying conditions for NEC, which might explain why premature infants are more susceptible to NEC than term infants are.
Cow’s milk protein might cause an immunomodulatory effect, for instance an increased production of TNF-α, which subsequently may result in a disturbed outgrowth of these intestinal epithelial stem cells with consequently an inflammatory bowel condition. Some evidence was provided by small studies suggesting that an exclusive human milk diet would results in lower NEC rates [34]. A recent randomized controlled trial, however, did not suggest that cow’s milk protein exposure resulted in a higher rate of NEC, when compared to pasteurized donor milk provided in the first 10 days of life [35].
Thus, most likely, several factors present, especially in unpasteurized milk, exert a beneficial effect, although results from the available high-quality studies are not overwhelming. Schanler et al. [36] did not find an effect on NEC in their blinded randomized controlled trial, but the most recent blinded randomized controlled trial did find a significant association between donor milk and the incidence of NEC [37]. These developments, the cost-effectiveness of human donor milk [38], and the potential benefits in the long term will probably lead to a policy where the use of preterm formula will only be limited to those infants of whom the parents do not want to use donor milk [39–41]. New techniques of pasteurization will in- crease the effectiveness of donor milk [42].

Fig. 2. Human Milk Center in Amsterdam 70 years ago.

Fig. 3. Present human milk bank in Amsterdam, The Netherlands.
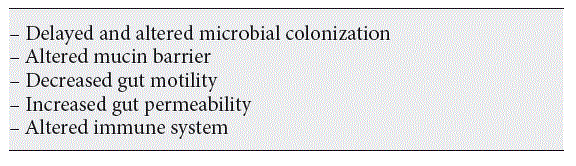
Table 1. Contributing factors to the increased risk of preterm infants developing NEC
Fortification of Human Milk
With the knowledge that human milk reduces NEC and nosocomial infections and the fact that nutrient requirements are not met with human milk alone, the usage of fortifiers became increasingly popular at the end of the 20th century. Variability in the composition of human milk and the high nutrient demands of preterm infants was acknowledged and commercial fortifiers became in vogue.
The first study on the use of human milk fortification was published in 1986 by Modanlou et al. [43]. They com- pared small groups of infants fed either preterm human milk (n = 10), fortified preterm human milk (n = 8), or premature formula (n = 12) and concluded that weight gain rates were similar in the infants fed preterm formula and fortified human milk, but infants fed preterm mother’s milk had lower rates of weight gain. Fourteen high- quality studies followed and current practice is that preterm infants receive fortified human milk. The observation is that fortification leads to higher in-hospital growth, but there are no effects on growth or neurodevelopment observed beyond infancy [44]. As time will proceed, not only cow’s milk proteins, vitamins, and minerals are supplied, but also fat and one might think of specific bioactive products. Lactoferrin is such a promising substance that might be added to the fortifiers in the future [45].
Probiotics
The knowledge that human milk contains bacteria originates from the 1950s [e.g., 46]. The first study that showed an effect of adding specific strains to preterm formula originated in the previous century [47]. Prior to that study, some reported the effect of adding probiotics on the microbiome [48–50]. Despite many trials that followed, and an overall clear effect of probiotic supplementation on reducing NEC incidence, probiotics were not used at all units. The heterogeneity of organisms and dosing regimens studied have prevented a species-specific treatment recommendation from being made so far [51– 54]. Furthermore, quality control of the available products remains an issue [55].
In conclusion, tremendous developments, both technological and nutritional, have been made, enabling higher survival rates but also higher quality of life. The revival of the use of human milk in the NICU will affect both these outcomes, certainly when the appropriate additives will be used.