Nutrition and intestinal microbiome
Setting the course for health:
Various research approaches commit themselves to functions and possible disorders of the infant intestinal microbiome. It is evidently an important control centre for development and health, the impact of which goes way beyond childhood. To what extent does nutrition influence this impact – and how can it be optimised?
Microbiome and exclusive breastfeeding
The CHILD Study is a prospective longitudinal cohort study, which guides almost
3,500 families across Canada right from the process of pregnancy to early childhood. In this study, the extent to which genetic and environmental factors influence the development and health of the child is being investigated. One focal point of the study is the connection between breastfeeding with the characteristics of the intestinal microbiome and their long-term outcome.
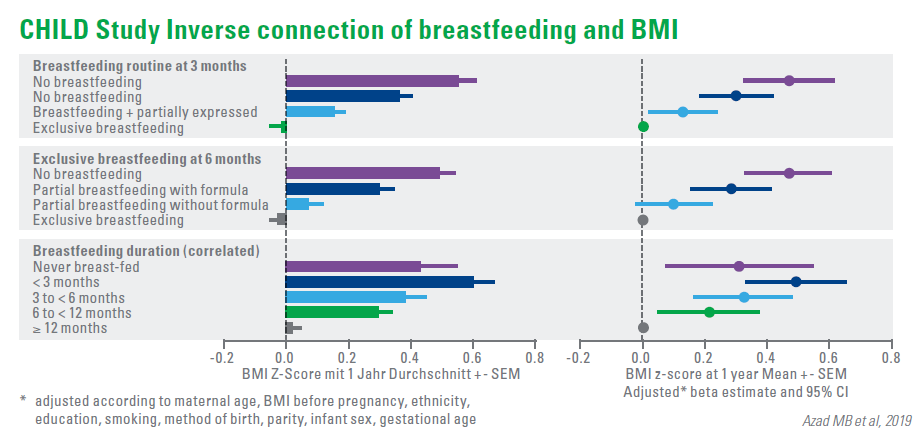
The latest analysis of the data of 2,553 infants demonstrated a dose-dependent inverse association between breastfeeding and body mass index (BMI) at 1 year of age. 1) Supplementing the diet with an infant formula in the 6th month has reduced the effect of breastfeeding, while the implementation of the supplementary diet had no significant effect. The study also revealed that infants, who received expressed breast milk in the first 3 months, had a higher BMI after 1 year than the breast-fed infants.
Evidently, there is also a connection between asthma risk and breastfeeding. Amongst the infants, whose mothers are suffering from asthma, exclusive breastfeeding was related to a reduced risk of wheezing within the first year of the infant’s life by 62%. The effect of breastfeeding on obesity and asthma is possibly related to the effects of breastfeeding on the intestinal microbiome of the infant.
Several components of breast milk, including microbiota and HMO, contribute to the positive effects of breastfeeding. The relative incidence of bifidobacteria was the highest in exclusively breast-fed infants and the lowest in the infants, who were not breast-fed. Other factors of breastfeeding, such as self-regulation and the intense mother-child bonding, can probably affect the results as well.
Impact factors on the microbiome
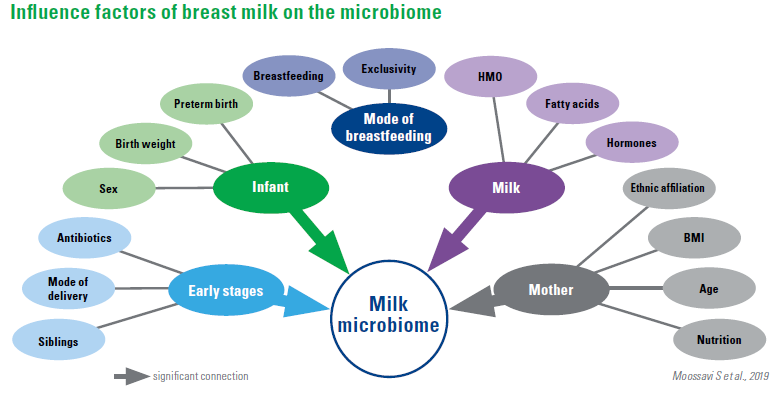
Breast milk contains a complex community of bacteria, which can help in enhancing the gut microbiota of the infant. The determinants for the composition of milk microbiota are still not very well known.
A study with 393 mother-children pairs from the Canadian CHILD cohort showed that the milk microbiome at 3-4 months postpartum was dominated by Proteobacteria and Firmicutes and had discrete compositional patterns. The composition and diversity were associated with
sex of the infant
maternal BMI
parity mode of delivery
breastfeeding practices
other milk components
The mode of breastfeeding proved to be one of the key determinants of milk microbiota composition. On the other hand, providing pumped breast milk showed enrichment of potential pathogens and depletion of bifidobacteria. Overall, this study offered an insight into the composition and essential determinants of the human milk microbiota composition, with potential implications for infant health and development.
According to the current notion, the application of one or multiple probiotic bacterial strains is a potential microbiome modelling treatment. The authors also offer an overview and the assessment of the relevant specialist publications.
A great biodiversity of microorganisms within the microbiome is increasingly being considered as a measure of a healthy microbiome. In the initial days of the infant’s life after birth, the intestinal microbiome is characterised by a low diversity, which consists of the diversity similar to that of an adult only at the age of 2 to 3 years. A reduced diversity of microbial colonisation is often related to illnesses.
Probiotics are understood to be non-pathogenic, living bacteria, which survive the gastrointestinal passage in sufficient numbers and contribute to improving or maintaining one’s health. The majority belongs to the group of lactic acid bacteria, including lactobacillus and bifidobacteria.
A uniform, probiotic mode of action is not currently known, but a variety of health-promoting interaction mechanisms of the microbiome, which consequently seem “probiotic”, have been described. The prophylactic administration of probiotics for chronic diseases could therefore be of great importance. For some disorders of the gastrointestinal tract, probiotics represent a potential therapeutic intervention. In many cases, however, the effectiveness of the probiotics appears to depend on the bacterial
strain used, the dose and the disease entity.
The authors’ conclusion:
In several cases, probiotics represent a harmless treatment option, which might
not be promising in all cases, however. Advancements in microbiome research and a better understanding of the interaction of individual microorganisms and molecular mechanisms of action will enhance the indication of probiotic intervention in the future.
What benefits does probiotics supplementation have for the mother?
Infants receive bioactive components through breastfeeding, which form your microbiome and are simultaneously influenced by microbial factors of breast milk and by the surface of the breast. More recent studies have suggested the possibility of an entero-mammary pathway of the microbial transfer, which opens up the possibility of a modulation of the infant intestinal microbiome through probiotic nutritional supplementation of the mother.
Breast milk samples were analysed, which were collected 10 days and 3 months postpartum from women, who participated in the placebo-controlled Trondheim study “Probiotics in the Prevention of Allergy among Children”. The women received a fermented milk, which was supplemented with Lactobacillus rhamnosus GG, Lactobacillus acidophilus La-5, and Bifidobacterium animalis ssp. lactis Bb-12. The application took place daily from 4 weeks before the expected date of delivery to 3 months after the birth. In total, 472 breast milk samples were analysed for the administered bacteria, and the microbiome transferred during breastfeeding was analysed for 142 samples.
It was found that breastfeeding is unlikely to be a significant source of L. rhamnosus GG, L. acidophilus La-5, and B. animalis ssp. lactis Bb-12 for infants in the probiotic arm of the trial. Furthermore, maternal supplementation did not significantly affect the overall composition of the breast milk microbiota transferred during breastfeeding. Samples collected at 3 months postpartum had a statistically significant lower presence and relative abundance of the Staphylococcus genus. These samples also had a greater number of observed species and diversity, including more operational taxonomic units from the Rothia, Veillonella, Granulicatella, and Methylbacterium genera.
HMO composition of breast milk in case of premature birth
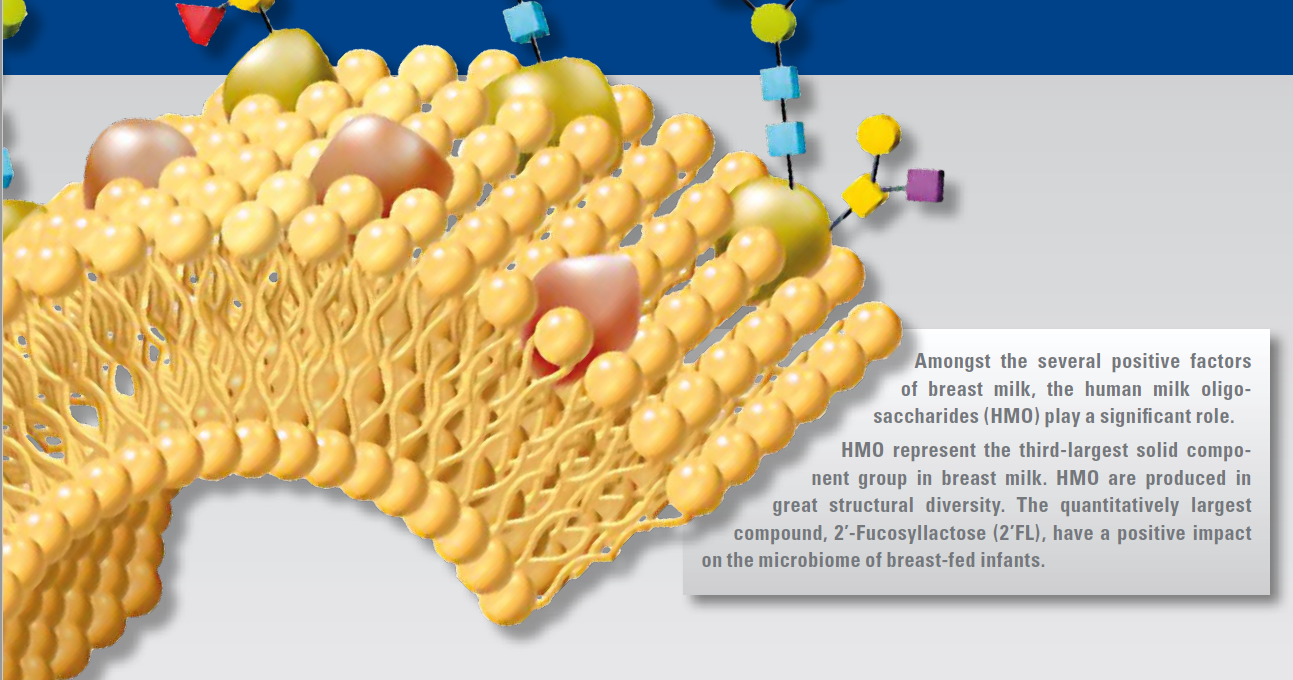
Oligosaccharides (HMO) are a primary component of breast milk and play an important role in protecting the infant against infections. Preterm infants are particularly susceptible, but have a better outcome if they are fed breast milk.
Randomised placebo-controlled trials with HMO supplementation indicate that 2′FL is related to the immune protection of infants and 2′FL together with Lacto-N-neotetraose (LNnT) is related to a protection of the lower respiratory tract against infections and a reduction of the necessity of the use of antibiotics.
Disialyllacto-N-tetraose (DSLNT) and 2′FL have turned out to prevent necrotizing enterocolitis (NEC) in rat models. In preterm infants, who develop NEC, DSLNT was present in lower concentration in the milk of their mothers. DSLNT could therefore be used as a marker for the probability of NEC development.
Fucosyltransferase-2 (FUT2) is responsible for binding fucose to core oligosaccharides
via a α-1.2 linkage, which generates HMO such as 2′-Fucosyllactose (2′FL) or Lacto-N-Fucopentaose-I (LNFP-I). If FUT2 is inactive, such HMO are not present in the milk. Fucosyltransferase-3 (FUT3), binds fucose to the key oligosaccharides via a α-1.4 linkage and forms oligosaccharides such as LNFP-II and, together with other, fucosyltransferase HMO via α-1.3 linkages. If FUT3 is inactive, the HMO with α-1.4 connections are not present in the milk. This results in four primary milk groups with different HMO compositions depending on the activity of the enzymes FUT2 and FUT3:
Milk group 1, in which both enzymes are active
Milk group 2, in which the FUT3 is active but not FUT2
Milk group 3, in which the FUT2 is active but not FUT3
Milk group 4, in which both enzymes are inactive
In general, milk group 1 seems to be the most common one, milk group 4 rare, but the distribution of the milk groups in different populations varies according to the genetic background. Based on the HMO composition of different milk samples, there are also indications that other sup-groups could exist.
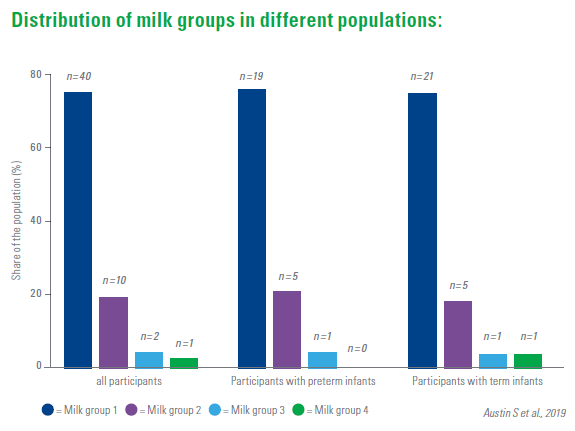
The trial investigated whether the HMO composition of breast milk of mothers of extremely young preterm infants (< 32 week of pregnancy, < 1500 g birth weight) differs from that of mothers of term babies. For this purpose, 22 different HMO in 500 milk samples of 25 mothers of preterm infants and of 28 mothers of term infants were analysed. In the same postpartum age, the concentrations of most HMO were similar.
In the same post-menstrual age, however, the concentrations of a series of HMO in the milk of mothers of preterm infants differs significantly from the concentrations in the milk of mothers of full-term infants. The biggest differences were seen in around 40 weeks after the birth (post-menstrual age), when the milk of the mothers of infants born close to the expected delivery date contained the highest concentrations of HMO. As regards their possible clinical effects, these findings require further investigations.
