Microbiota and Neurodevelopmental Trajectories - Role of Maternal and Early-Life Nutrition
Key Messages
- The microbial trajectory across pregnancy and early life coincides with key neurodevelopmental periods.
- Diet, drugs and stress modulate early-life microbial colonization.
- Early-life interventions with prebiotics and probiotics could modulate the microbiota and neurodevelopment.
Keywords
Microbiota · Neuropsychiatry · Gut-brain axis · Brain development · Early life · Stress · Diet · Nutrition
Abstract
Pregnancy and early life are characterized by marked changes in body microbial composition. Intriguingly, these changes take place simultaneously with neurodevelopmental plasticity, suggesting a complex dialogue between the microbes that inhabit the gastrointestinal tract and the brain. The purpose of this chapter is to describe the natural trajectory of microbiota during pregnancy and early life, as well as review the literature available on its interaction with neurodevelopment. Several lines of evidence show that the gut microbiota interacts with diet, drugs and stress both prenatally and postnatally. Clinical and preclinical studies are illuminating how these disruptions result in different developmental outcomes. Understanding the role of the microbiota in neurodevelopment may lead to novel approaches to the study of the pathophysiology and treatment of neuropsychiatric disorders.
Introduction
The connection between the brain and the gastrointestinal tract has been extensively studied, but the existence of a bidirectional microbiota-gut-brain axis has only received attention in the last decade [1, 2]. The individual microorganisms that live in our body, the microbiota, and their collective genomes, the microbiome, exert considerable influence over host brain and behaviour [3, 4] (Table 1). Variations in microbiota composition have been linked to neuropsychiatric disorders, including autism, stress, anxiety and major depressive disorder [3, 5].
Almost 30 years ago, it was proposed that prenatal and postnatal environmental factors interact with genetics to program health and disease in adulthood [6, 7]. Building on Barker’s hypothesis, it was recently proposed that the microbiota could play an important role in programming adult brain health and disease [8]. Whether diet or other factors, such as stress and drugs, interact with the microbiota in early life to program brain health is currently being addressed by clinical and preclinical studies. This chapter reviews the natural trajectory of the composition of the microbiota during pregnancy and early life and outlines the current knowledge on the interaction be- tween the microbiota and neurodevelopment.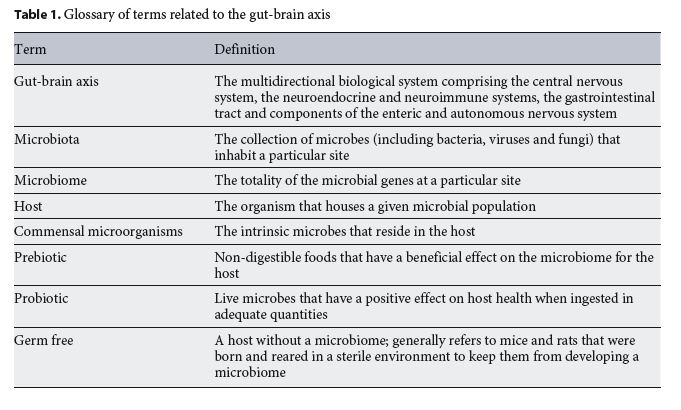
Early-Life Neurodevelopmental Plasticity and the Microbiota
Dramatic structural and functional changes in the brain are characteristic of the first years of life. This neurodevelopmental plasticity requires timely and adequate migration, division and differentiation of neuronal and glial precursors [9]. Neuronal migration and axonal guidance establish short- and long-range connections that enable the recruitment of multiple brain areas for the execution of complex behaviours [10, 11]. Differentiated oligodendrocytes insulate neuronal axons with a myelin sheath to guarantee proper conductance of neuronal signals [12]. A growing emphasis is now placed on the role of astrocytes and microglia in facilitating synaptic pruning during early life through adolescence, allowing later in life the fine tuning of complex circuits [13]. Plasticity is a key feature of the standard neurodevelopmental trajectory and modulates the dynamics of synaptic connections and neural circuitry formation. Deviations from the neurodevelopmental trajectory can account for increased susceptibility to brain diseases later in life.
There is a growing appreciation of the link between neurodevelopment and intestinal microbiota. Studies in germ-free mice have shown abnormal brain development, especially in male mice [14–16]. More recent studies in these microbiota-deficient mice have shown altered expression of genes implicated in neurophysiology processes, such as neurotransmission, neuronal plasticity, metabolism and morphology in the amygdala [17] and hippocampus [18]. Hypermyelination in the pre-frontal cortex and abnormal microglia maturation characterize the glia profile of these animals [19–23]. Furthermore, they showed increased blood-brain barrier permeability [24]. Functionally, such changes translate to increased stress response [14, 16], changes in anxiety [25] and fear recall [26], cognitive deficits [27], social changes [21, 28] and visceral pain responses [29]. Thus, the complete absence of microbial colonization in early life has dramatic effects on offspring’s brain development and function.
Dynamics of the Maternal Microbiota during Pregnancy
Pregnancy is a unique period in human life, and both the gut and vaginal microbiome have evolved to follow an optimum trajectory to support the mother and the developing fetus and allow for the ideal handover of microbiome at birth, informing maternal and child health outcomes.
The human female gut microbiota undergoes dynamic compositional changes across gestation [30–32]. As pregnancy progresses, a reduction in the diversity of the intestinal microbiota takes place, characterized by an enrichment in Proteobacteria [30]. This natural shift in the bacterial populations is functional to the increased metabolic demands by the developing fetus. The Proteobacteria expansion can help the body with the increased energetic requirement that is characteristic of the third trimester [33]. Interestingly, when gut microbiota from this time period was transferred to microbiota-depleted rats, they showed increased adiposity, reduced glucose tolerance and inflammation, signs of metabolic syndrome [30]. This suggests that the changes in gut microbiota composition during pregnancy have an adaptive role for maternal and newborn health.
The vaginal microbiota composition also changes during pregnancy towards a less diverse configuration [34, 35]. As with gastrointestinal microbiota, the change in vaginal microbiota has a specific role during pregnancy. An in- crease in the presence of Lactobacilli helps maintain a low pH, limiting bacterial growth opportunity for other bacteria [35]. Furthermore, vaginal microbiota composition is critical in the context of vertical transmission of microbial populations [36]. Whether interventions in the physiological trajectory of maternal microbiota could alter the prenatal environment and, in turn, deviate normal brain development is a key question in neuroscience that is starting to be addressed both in preclinical and clinical areas.
Preclinical Models of Early-Life Microbiota Trajectory
Similar to humans, mice and rat intestinal and vaginal microbiota go through compositional changes during pregnancy, providing a robust preclinical model for studying the link between maternal gut environment and offspring brain development [37–40]. Early gestation is characterized by a transitional increase in the relative abundance of Akkermansia and Bifidobacterium, which in late pregnancy decrease to levels seen in non-pregnant mice. In contrast, Bacteroides remain relatively elevated throughout pregnancy [37]. Interestingly, microbiota compositional changes also occur post-partum. The relative abundance of Actinobacteria increases early post-partum, while the one of Bacteroidetes decreases [38].
The vaginal microbiota has its own trajectory in pregnant mice. After the first week of pregnancy, there is an increase in bacterial diversity characterized by a growth of the Firmicutes and Bacteroidetes phyla [40, 41]. The changes seen in mice gut microbiota during pregnancy and post-partum make it a solid approach to the study of interventions in the maternal microbiota and the impact on offspring’s neurodevelopment.
External Challenges to Maternal Microbiota Dynamics
Given the importance of early-life microbiota in neurodevelopment, any factor that affects its composition has the potential to influence brain health. Indeed, a variety of exogenous factors affect the trajectory of microbiota composition during pregnancy. Diet, drugs, infection, hospitalization, prematurity and stress are among the influences that divert maternal microbiota from its natural course and impact on offspring’s brain, immune system and the hypothalamic-pituitary-adrenal axis (HPA) development.
Diet and Maternal Microbiota
Diet is one of the major sculptors of the diversity and abundance of the intestinal microbiota [42]. Inadequate intake of macronutrients or micronutrients during pregnancy has been related to altered maternal microbiota [43] and offspring’s poor neurocognitive outcome (Table 2) [44]. This association suggests a role for the maternal microbiota in brain prenatal programming.
One of the most common macronutrient consumption imbalances during pregnancy is the consumption of high-fat diets. Maternal overweight has been associated in humans with increased risk of poor neurodevelopmental outcomes [45]. In rodents, consumption of a high-fat or Western diet prior and during pregnancy impairs the trajectory of maternal and offspring’s microbiota [37, 46]. This alteration was associated with a neuroinflammatory profile in the hippocampus and amygdala of the offspring, resulting in juvenile impaired social behaviour and anxiety-like phenotype [47]. Interestingly, a high-fat diet prior to and during pregnancy impairs maternal HPA axis plasticity and the offspring’s hypothalamic gene response to stress [48, 49]. However, caution is required when interpreting the literature on the neurobiological changes induced by diets rich in fat and sugar in rodents as the content of the control diets regarding fibre and other nutrients needs to be taken into account [50, 51]. Nevertheless, preclinical studies on maternal high-fat and Western diets (see [8] for an extensive review) support the idea of a role for diet-induced microbiota changes in brain programming.
During fetal development, micronutrients are required for neurological development. Deficiency in B vitamins, folate or ions, such as iron and zinc, exerts detrimental effects on neurocognitive development in humans and rodents [52, 53]. Folate deficiency is paradigmatic of the impact of micronutrient deficit on offspring neurodevelopment. Mammalian cells are unable to synthetize this vitamin; thus, humans depend on food or supplements to compensate for their requirement [54]. Failure to achieve normal serum folate levels during pregnancy has been associated with increased neural tube defects in the off- spring [55]. Conveniently, bacteria residing in our colon can produce many vitamins of the B group, including folate. In mice, a loss-of-function mutation in an intestinal folate transporter can account for folate malabsorption, suggesting that bacterial produced folate plays a major role in host metabolism [56]. In humans, consumption of a vegetarian diet during early pregnancy was associated with a distinctive microbial composition rich in biosynthesis pathways for fatty acids, lipids and folate [57].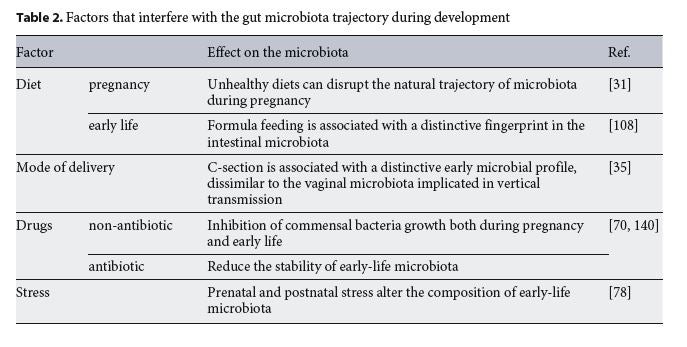
Prebiotics and Probiotics
Research on the effect of prebiotic and probiotic administration during pregnancy is at an early stage (Table 3). Current reports indicate that the administration of prebiotics or probiotics to pregnant women is not associated with an increase or decrease in the risk of preterm birth or other infant and maternal adverse pregnancy outcomes [58]. Researchers are beginning to shed light on their effects on offspring’s brain and immune development [58].
Prebiotics promote the growth of beneficial bacteria and include indigestible fibres that are fermented by colonic bacteria to produce short-chain fatty acids and provide a health benefit [59]. In humans, the effects of maternal intake of prebiotics on neurodevelopment have not been well studied, and there is uncertainty about their effects on allergy risk [60, 61]. Galacto-oligosaccharide (GOS) and inulin administration to pregnant mice modulated the gut microbiota and prevented immune activation and intestinal permeability in the offspring [62]. More-over, it has recently been shown that the addition of inulin to a mouse maternal high-fat diet abrogated the negative metabolic effect of the high-fat diet on offspring [63].
Probiotics are beneficial strains of bacteria that confer a health benefit to the host [64]. There is lack of research on the prenatal impact of probiotics on neurodevelopment in humans and rodents. Administration of probiotics to pregnant women impact on immunity, reducing the risk of atopy but not of asthma [65, 66]. More preclinical and clinical research must be conducted to determine the impact of prenatal probiotics on the maternal and off- spring microbiota.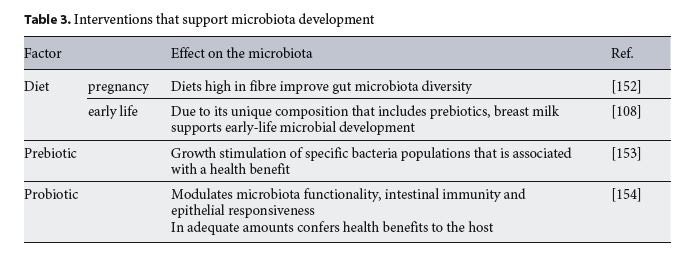
Drugs
Antibiotics
Antibiotics are widely used during pregnancy, but little is known about their effects on the trajectory of the maternal microbiome [67]. Preclinical models are starting to shed light on the effect of antibiotic exposure on offspring neurodevelopment. Administration of antibiotics to pregnant rats caused impairments in social behaviour and pre-pulse inhibition of the offspring [68]. In mice, administration of non-absorbable antibiotics during pregnancy reduced the exploratory behaviour in the offspring [69]. These results warrant further research on the effect of microbiota.
Psychotropics
Recently, Maier et al. [70] showed that a large amount of non-antibiotic human-targeted drugs have antimicrobial properties. Among them, drugs that can be prescribed during pregnancy, such as proton pump inhibitors, were found to disturb the growth of commensal bacteria (Table 2). Interestingly, psychotropic medications also influence the composition of gut bacteria in rodents [70, 71]. Selective serotonin uptake inhibitors, tricyclic antidepressants and antipsychotics negatively impact bacterial growth [71–73]. Looking at the effects on post- natal development, prenatal exposure to fluoxetine induces an anxiety-like phenotype in rats [74]. Also, in rodents, valproic acid administration during pregnancy disturbs the microbiome of the offspring and results in impairment of the social behaviour of the offspring [75,76]. Owing to the prevalence of psychotropic administration during pregnancy, it is crucial to characterize the interaction between maternal health, microbiota and off- spring neurodevelopment.
Stress and the Maternal Microbiota
In humans, prenatal and postnatal maternal stress has been associated with young adult offspring behavioural and depressive symptoms [77] and aberrant infant intestinal microbiota development (Table 2) [78, 79]. In rodents, prenatal stress shifts maternal gut and vaginal bacterial community and induces long-lasting alterations in the gut microbiota composition of the offspring [40, 80]. Moreover, this alteration was shown to occur in a sex- specific manner, and it correlates with hyper-reactivity of the HPA axis [40].
The Microbiota in Transition: from Prenatal to Postnatal
When the first contact with the microbiota occurs re- mains controversial. The sterility of the uterus during pregnancy is one of the paradigms that research on the microbiome is revisiting. Bacteria have been found in the placenta [81, 82], amniotic fluid and meconium of humans [83, 84]. Moreover, the presence of specific bacteria in utero has been associated with pregnancy risks, including higher rates of preterm delivery [85]. Nevertheless, the reliability of these findings is widely debated in the context of whether it is contamination or not [86, 87]. The existence of germ-free mice models further dismisses the idea of a prenatal microbiome [86]. It is generally accepted that the moment of birth is the first opportunity for large-scale bacterial colonization of the newborn. Thus, the mode of delivery has a tremendous impact on the establishment of the microbiota of infants.
Early-Life Microbiota and Birth Mode
A large number of studies associate the mode of delivery to a distinctive trajectory of microbiota development in the newborn [35, 36, 66, 88–99]. Unexposed to the birth canal, Caesarean section (C-section)-born babies elude mother-neonate vertical vaginal transmission of bacteria and viruses [36, 89, 100]. In turn, the microbiota resembles skin and environment microbiota, suggesting that C-section first colonizers come from diverse sources (Table 2) [35, 89].
That said it is worth reinforcing that mode of delivery- induced changes in microbiota composition are transitory. Vaginally delivered infants have significantly higher microbiota richness and diversity than C-section-born infants as early as 3 days after birth [88, 100–102]. Nevertheless, the early decline in Proteobacteria and the late Firmicutes expansion occur timely over the first year of life of C-section-born infants [101].
The time course of these microbiota alterations overlaps with a critical period for neuro- and immune development (see [103] for extensive review). It has been suggested that C-section-distinctive microbiota composition plays a functional role in predisposing these infants to a greater relative risk of neonatal infections, allergy, asthma, obesity and type 1 diabetes [35, 101, 104–108]. Pre-clinical models of C-section suggest that the mode of delivery could impact on early neuronal maturation [109, 110]. Whether modifying the initial colonizing microbiota induces directly or indirectly different trajectories in brain development has yet to be deciphered.
Epidemiology studies have shown that C-section-induced changes in terms of brain health and school performance later in life are subtle at best [111, 112] and, in the case of autism, do not withstand correcting for familial confounding [111].
Various strategies have been designed to restore the normal trajectory of the microbiota [113]. Although controversial, artificial vaginal microbiota transference was performed to C-section-born infants to mimic vertical transmission [114]. Other interventions, including supplementation with probiotics and prebiotics, were proposed to decrease the impact of delivery mode on the microbiota.
Early Postnatal Perturbations of the Microbiota
Early postnatal life entails an intrinsic sensitivity to environmental factors. As with the maternal microbiome, infant exposure to differences in diet, drugs and stress can interfere with the trajectory of the microbiota and neuro-development in a manner that is characteristic of this developmental period.
Mode of Nutritional Provision in Early Life
The stability and composition of the early-life gut microbiota community is also dependent on diet [115]. Ac- cumulating evidence suggests that breastfeeding and formula-based nutrition leave a distinctive fingerprint in the intestinal microbiota (Table 2). Gut bacterial composition of infants exclusively breastfed is characterized by higher relative abundance of Bacteroides and Bifidobacterium compared to the one from formula-fed infants [108, 116]. Furthermore, breastfeeding had a positive effect on myelination and increased general, verbal and non-verbal cognitive abilities during childhood [117]. The implications of these findings are still unclear, but longitudinal studies are starting to shed light on the effect of early-life nutrition on the temporal course of microbiota maturation.
Human breast milk has a unique composition that interacts with the developing gut microbiota. Culture-dependent and -independent techniques revealed that it is a source of bacteria [118]. Interestingly, the human milk microbiome can be influenced by maternal body mass index and mode of delivery [119]. The other main components of breast milk are human milk oligosaccharides, which act as prebiotics [120, 121]. Supplementation of infant formula with GOS increases the abundance of Bi- fidobacteria and Lactobacilli to levels reported in breast-fed infants [122, 123]. Both breast milk microbes and prebiotics play a role in the standard gut microbial develop- mental trajectory.
Later in life, feeding transitions drive important changes in composition and functionality of the intestinal microbiota [36, 89, 124]. From breastfeeding to solid food, the microbiome transitions from being enriched in genes associated with digestion of sugars from breast milk, vitamin production and iron transport to degradation of starch and high sugars [36]. Further-more, the microbiota continues to undergo change; at 7–12 years of age, the composition and function of the microbiota remains significantly different from the one of adults [125], suggesting a role of the microbiome in the neurodevelopmental changes associated with adolescence.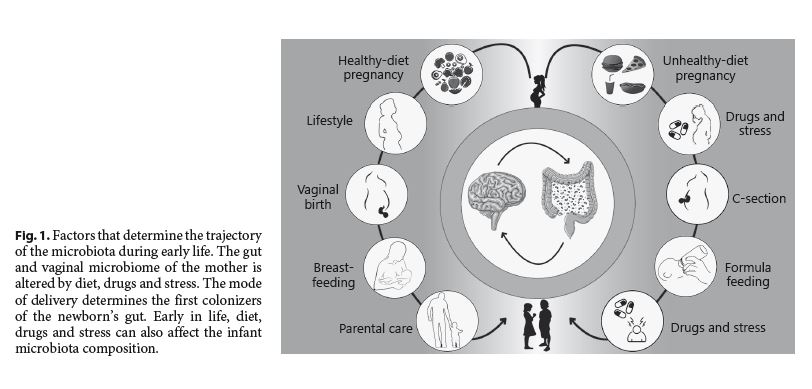
Probiotics and Prebiotics
Most of the evidence available on the effect of early-life exposure to pre-and probiotics comes from preclinical studies. Early-life prebiotic administration in humans has shown effects on reducing the risk of atopy, an autoimmune disease [126], but neurodevelopmental outcomes have not been studied yet. In preclinical studies, oligosaccharides have been shown to modulate the gut-brain axis, highlighting the role of breastfeeding in neurodevelopment. Administration of the human milk oligosaccharides 3’Sialyllactose (3’SL) or 6’Sialyllactose (6’SL) to mice exposed to social disruption prevented stress-induced colonic microbial disruption and anxiety-like behavior [127]. Furthermore, fructo-oligosaccharide (FOS) and GOS administration attenuated corticosterone release in response to an acute stressor and protected the mice from the impact of chronic stress on the microbiota [128].
Preliminary clinical trials of probiotic interventions have yielded promising results with regard to reducing the risk for gastrointestinal problems, sepsis, allergies and even autism spectrum disorder and attention deficit hyperactivity disorder [129–134]. Several groups have now shown that early probiotic interventions mitigate the effects of early-life stress, maternal high-fat diet and maternal immune activation on infant outcomes [47, 135–138]. Oral administration at weaning of Bifidobacterium fragilis ameliorates the abnormal stereotyped and anxiety-like behaviours of the maternal immune activation mouse model of autism [136]. Probiotic administration during adolescence restores social interaction-induced long- term potentiation in an animal model of social impairment by maternal high-fat diet exposure [47]. In maternally separated rat pups, a combination of Lactobacillus rhamnosus and Lactobacillus helveticus reduced pup corticosterone responses to stress and normalized fear behaviour [135, 137, 138]. Another probiotic, Bifidobacterium infantis, normalized behavioural deficits in adult rats exposed to maternal separation [139].
Although clinical evidence on the role of pre-and probiotics for neurodevelopment is still lacking, preclinical research gives cause for a focus on early-life microbiota interventions.
Drugs: Antibiotics and Beyond in a Paediatric Setting
Antibiotics are commonly prescribed during the first years of life, yet the effect on brain health programming is unknown. Longitudinal clinical studies support the idea that early-life exposure to antibiotics perturbs the natural trajectory of the microbial communities by altering their stability [140]. Furthermore, neonatal exposure to antibiotics in rodents not only altered the microbiota but also induced increased visceral sensitivity and long-lasting changes in brain cytokines and behaviour [141, 142].
The interaction between early-life exposure to psychotropics, neurodevelopment and the microbiota is currently unknown. Not only exposure to psychotropics mediated by breastfeeding but direct administration of these drugs early in life could impact the developing microbiota. Serotonin uptake inhibitors and atypical antipsychotics indicated for the treatment of paediatric psychiatric disorders are among the non-antibiotic drugs known to change the microbiome composition [70, 71]. Atypical antipsychotics indicated for the treatment of the irritability associated with autism spectrum disorders have been shown to inhibit gut bacteria [70]. At the same time, the composition of the microbiota of autistic patients was shown to be altered [143–147]. Whether there is an interaction between microbiota populations, psychotropic drugs and behaviour has yet to be determined.
Early-Life Stress
The impact of stress on the development of the HPA axis has been shown to contribute to the programming of brain health in later life [148]. Interestingly, evidence from preclinical studies shows that early-life stress also alters the microbiota. Maternal separation during early life disrupted the microbiota of the offspring of rhesus monkeys and rats [149, 150]. Interestingly, a diet containing prebiotics in combination with live Lactobacillus rhamnosus GG attenuated the effects of early-life maternal separation on anxiety-like behaviour and hippocampal-dependent learning [151]. Germ-free mice were more vulnerable to restraint stress, resulting in higher adrenocorticotropic hormone and corticosterone in plasma [14, 16], a reduction in glucocorticoid receptor mRNA and an increased stress response [14]. Remarkably, these effects were rescued with microbiota transplantation during adolescence but not adulthood [14].
Future Perspectives
Pregnancy and the first years of life are unique stages of plasticity for the intestinal microbiota. In both cases, there is a dynamic trajectory of the intestinal microbiota composition that is functional to the requirements of the host. Although plasticity represents an opportunity for adaptation, it is also a vulnerable stage. As we have reviewed, clinical and preclinical studies suggest that diet, stress and drugs can interact with the natural trajectory of the microbiota and play a part in programming brain health (Fig. 1). However, the evidence is still scarce, and further research is needed to understand the functional implications of these interactions.
The nervous system and the microbiota show concurrent developmental trajectories, offering a unique opportunity for intervention. There is potential for the development of early-life-targeted interventions of the microbiome, aiming to reduce the risk of disease later in life. Further research is needed on the characterization of critical windows to modulate the microbiota and the consequences of early intervention.
References
1 Foster JA, McVey Neufeld KA. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013 May; 36(5):305–12.
2 Bienenstock J, Kunze W, Forsythe P. Microbiota and the gut-brain axis. Nutr Rev. 2015 Aug;73 Suppl 1:28–31.
3 Dinan TG, Cryan JF. Gut instincts: microbiota as a key regulator of brain development, ageing and neurodegeneration. J Physiol. 2017 Jan;595(2):489–503.
4 Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012 Oct;13(10):701–12.
5 Vuong HE, Hsiao EY. Emerging roles for the gut microbiome in autism spectrum disorder. Biol Psychiatry. 2017 Mar;81(5):411–23.
6 Barker DJ. The developmental origins of adult disease. J Am Coll Nutr. 2004 Dec;23(6 Sup- pl):588S–95S.
7 Barker DJ, Gluckman PD, Godfrey KM, Harding JE, Owens JA, Robinson JS. Fetal nutrition and cardiovascular disease in adult life. Lancet. 1993 Apr;341(8850):938–41.
8 Codagnone MG, Spichak S, O’Mahony SM, O’Leary OF, Clarke G, Stanton C, et al. Programming bugs: microbiota and the developmental origins of brain health and disease. Biol Psychiatry. 2019 Jan;85(2):150–63.
9 Forrest MP, Parnell E, Penzes P. Dendritic structural plasticity and neuropsychiatric disease. Nat Rev Neurosci. 2018 Mar;19(4):215– 34.
10 Rodier PM. Environmental causes of central nervous system maldevelopment. Pediatrics. 2004 Apr;113(4 Suppl):1076–83.
11 Fornito A, Zalesky A, Breakspear M. The connectomics of brain disorders. Nat Rev Neuro- sci. 2015 Mar;16(3):159–72.
12 Fields RD. A new mechanism of nervous system plasticity: activity-dependent myelination. Nat Rev Neurosci. 2015 Dec;16(12):756– 67.
13 Hong S, Stevens B. Microglia: Phagocytosing to Clear, Sculpt, and Eliminate. Dev Cell. 2016 Jul;38(2):126–8.
14 Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN, et al. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol. 2004 Jul;558(Pt 1):263–75.
15 Neufeld KM, Kang N, Bienenstock J, Foster JA. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol Motil. 2011 Mar;23(3): 255–64.
16 Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney RD, Shanahan F, et al. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex- dependent manner. Mol Psychiatry. 2013 Jun; 18(6):666–73.
17 Stilling RM, Ryan FJ, Hoban AE, Shanahan F, Clarke G, Claesson MJ, et al. Microbes & neurodevelopment—absence of microbiota during early life increases activity-related transcriptional pathways in the amygdala. Brain Behav Immun. 2015 Nov;50:209–20.
18 Chen JJ, Zeng BH, Li WW, Zhou CJ, Fan SH, Cheng K, et al. Effects of gut microbiota on the microRNA and mRNA expression in the hippocampus of mice. Behav Brain Res. 2017 Mar;322 Pt A:34–41.
19 Hoban AE, Stilling RM, Ryan FJ, Shanahan F, Dinan TG, Claesson MJ, et al. Regulation of prefrontal cortex myelination by the microbiota. Transl Psychiatry. 2016 Apr;6(4):e774.
20 Erny D, Hrabě de Angelis AL, Jaitin D, Wieg- hofer P, Staszewski O, David E, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neuro- sci. 2015 Jul;18(7):965–77.
21 Desbonnet L, Clarke G, Shanahan F, Dinan TG, Cryan JF. Microbiota is essential for social development in the mouse. Mol Psychiatry. 2014 Feb;19(2):146–8.
22 Thion MS, Low D, Silvin A, Chen J, Grisel P, Schulte-Schrepping J, et al. Microbiome Influences Prenatal and Adult Microglia in a Sex-Specific Manner. Cell. 2018 Jan;172(3): 500–516.e16.
23 Gacias M, Gaspari S, Santos PM, Tamburini S, Andrade M, Zhang F, et al. Microbiota-driven transcriptional changes in prefrontal cortex override genetic differences in social behavior. Elife. 2016 Apr;5:e13442.
24 Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Tóth M, et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. 2014 Nov; 6(263):263ra158.
25 Diaz Heijtz R, Wang S, Anuar F, Qian Y, Björkholm B, Samuelsson A, et al. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci USA. 2011 Feb;108(7):3047–52.
26 Hoban AE, Stilling RM, Moloney G, Shanahan F, Dinan TG, Clarke G, et al. The microbiome regulates amygdala-dependent fear recall. Mol Psychiatry. 2018 May;23(5):1134– 44.
27 Gareau MG, Wine E, Rodrigues DM, Cho JH, Whary MT, Philpott DJ, et al. Bacterial infection causes stress-induced memory dysfunction in mice. Gut. 2011 Mar;60(3):307–17.
28 Sgritta M, Dooling SW, Buffington SA, Momin EN, Francis MB, Britton RA, et al. Mechanisms underlying microbial-mediated changes in social behavior in mouse models of autism spectrum disorder. Neuron. 2019 Jan;101(2):246–259.e6.
29 Luczynski P, Tramullas M, Viola M, Shanahan F, Clarke G, O’Mahony S, et al. Microbiota regulates visceral pain in the mouse. Elife. 2017 Jun;6:e25887.
30 Koren O, Goodrich JK, Cullender TC, Spor A, Laitinen K, Bäckhed HK, et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell. 2012 Aug; 150(3):470–80.
31 Collado MC, Isolauri E, Laitinen K, Salminen S. Distinct composition of gut microbiota during pregnancy in overweight and normal- weight women. Am J Clin Nutr. 2008 Oct; 88(4):894–9.
32 Aagaard K, Riehle K, Ma J, Segata N, Mistret- ta TA, Coarfa C, et al. A metagenomic approach to characterization of the vaginal microbiome signature in pregnancy. PLoS One. 2012;7(6):e36466.
33 Shin NR, Whon TW, Bae JW. Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015 Sep;33(9): 496–503.
34 Romero R, Hassan SS, Gajer P, Tarca AL, Fadrosh DW, Nikita L, et al. The composition and stability of the vaginal microbiota of normal pregnant women is different from that of non-pregnant women. Microbiome. 2014 Feb;2(1):4.
35 Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci USA. 2010 Jun;107(26):11971–5.
36 Hill CJ, Lynch DB, Murphy K, Ulaszewska M, Jeffery IB, O’Shea CA, et al. Evolution of gut microbiota composition from birth to 24 weeks in the INFANTMET Cohort. Microbiome. 2017 Jan;5(1):4.
37 Gohir W, Whelan FJ, Surette MG, Moore C, Schertzer JD, Sloboda DM. Pregnancy-related changes in the maternal gut microbiota are dependent upon the mother’s periconceptional diet. Gut Microbes. 2015;6(5):310–20.
38 Fuller M, Priyadarshini M, Gibbons SM, Angueira AR, Brodsky M, Hayes MG, et al. The short-chain fatty acid receptor, FFA2, contributes to gestational glucose homeostasis. Am J Physiol Endocrinol Metab. 2015 Nov; 309(10):E840–51.
39 Hallam MC, Barile D, Meyrand M, German JB, Reimer RA. Maternal high-protein or high-prebiotic-fiber diets affect maternal milk composition and gut microbiota in rat dams and their offspring. Obesity (Silver Spring). 2014 Nov;22(11):2344–51.
40 Jašarević E, Howard CD, Misic AM, Beiting DP, Bale TL. Stress during pregnancy alters temporal and spatial dynamics of the maternal and offspring microbiome in a sex-specific manner. Sci Rep. 2017 Mar;7(1):44182.
41 Jašarević E, Howerton CL, Howard CD, Bale TL. Alterations in the Vaginal Microbiome by Maternal Stress Are Associated with Metabolic Reprogramming of the Offspring Gut and Brain. Endocrinology. 2015 Sep;156(9):3265– 76.
42 Turnbaugh PJ, Bäckhed F, Fulton L, Gordon JI. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe. 2008 Apr;3(4):213–23.
43 Zacarías MF, Collado MC, Gómez-Gallego C, Flinck H, Aittoniemi J, Isolauri E, et al. Pre-gestational overweight and obesity are associated with differences in gut microbiota composition and systemic inflammation in the third trimester. PLoS One. 2018 Jul; 13(7):e0200305.
44 Monk C, Georgieff MK, Osterholm EA. Re- search review: maternal prenatal distress and poor nutrition – mutually influencing risk factors affecting infant neurocognitive development. J Child Psychol Psychiatry. 2013 Feb; 54(2):115–30.
45 Sanchez CE, Barry C, Sabhlok A, Russell K, Majors A, Kollins SH, et al. Maternal pre- pregnancy obesity and child neurodevelop mental outcomes: a meta-analysis. Obes Rev. 2018 Apr;19(4):464–84.
46 Steegenga WT, Mischke M, Lute C, Boek- schoten MV, Lendvai A, Pruis MG, et al. Maternal exposure to a Western-style diet causes differences in intestinal microbiota composition and gene expression of suckling mouse pups. Mol Nutr Food Res. 2017 Jan;61(1):61.
47 Buffington SA, Di Prisco GV, Auchtung TA, Ajami NJ, Petrosino JF, Costa-Mattioli M. Microbial Reconstitution Reverses Maternal Diet-Induced Social and Synaptic Deficits in Offspring. Cell. 2016 Jun;165(7):1762–75.
48 Perani CV, Neumann ID, Reber SO, Slattery DA. High-fat diet prevents adaptive peripar- tum-associated adrenal gland plasticity and anxiolysis. Sci Rep. 2015 Oct;5(1):14821.
49 Grissom NM, George R, Reyes TM. The hypothalamic transcriptional response to stress is severely impaired in offspring exposed to adverse nutrition during gestation. Neuroscience. 2017 Feb;342:200–11.
50 Almeida-Suhett CP, Scott JM, Graham A, Chen Y, Deuster PA. Control diet in a high-fat diet study in mice: regular chow and purified low-fat diet have similar effects on phenotypic, metabolic, and behavioral outcomes. Nutr Neurosci. 2019 Jan;22(1):19–28.
51 Pellizzon MA, Ricci MR. The common use of improper control diets in diet-induced metabolic disease research confounds data interpretation: the fiber factor. Nutr Metab (Lond). 2018 Jan;15(1):3.
52 Georgieff MK. The role of iron in neurodevelopment: fetal iron deficiency and the developing hippocampus. Biochem Soc Trans. 2008 Dec;36(Pt 6):1267–1271.
53 Gernand AD, Schulze KJ, Stewart CP, West KP Jr, Christian P. Micronutrient deficiencies in pregnancy worldwide: health effects and prevention. Nat Rev Endocrinol. 2016 May; 12(5):274–89.
54 Kok DE, Steegenga WT, McKay JA. Folate and epigenetics: why we should not forget bacterial biosynthesis. Epigenomics. 2018 Sep;10(9):1147–1150.
55 Milunsky A, Jick H, Jick SS, Bruell CL, Mac- Laughlin DS, Rothman KJ, et al. Multivita- min/folic acid supplementation in early preg- nancy reduces the prevalence of neural tube defects. JAMA. 1989 Nov;262(20):2847–52.
56 Qiu A, Jansen M, Sakaris A, Min SH, Chatto- padhyay S, Tsai E, et al. Identification of an intestinal folate transporter and the molecular basis for hereditary folate malabsorption. Cell. 2006 Dec;127(5):917–28.
57 Barrett HL, Gomez-Arango LF, Wilkinson SA, McIntyre HD, Callaway LK, Morrison M, et al. A vegetarian diet is a major determinant of gut microbiota composition in early pregnancy. Nutrients. 2018 Jul;10(7):890.
58 Jarde A, Lewis-Mikhael AM, Moayyedi P, Stearns JC, Collins SM, Beyene J, et al. Pregnancy outcomes in women taking probiotics or prebiotics: a systematic review and meta-analysis. BMC Pregnancy Childbirth. 2018 Jan;18(1):14.
59 Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, et al. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017 Aug;14(8):491–502.
60 Wang E, Vazifedan T, Hogan AD. Prebiotics for the prevention of allergies: A systematic review and meta-analysis of randomized controlled trials. Pediatrics. 2018; 142(Suppl 4):S209–10.
61 Cuello-Garcia C, Fiocchi A, Pawankar R, Yepes-Nuñez JJ, Morgano GP, Zhang Y, et al. Prebiotics for the prevention of allergies: A systematic review and meta-analysis of randomized controlled trials. Clin Exp Allergy. 2017 Nov;47(11):1468–77.
62 Bouchaud G, Castan L, Chesné J, Braza F, Aubert P, Neunlist M, et al. Maternal exposure to GOS/inulin mixture prevents food allergies and promotes tolerance in offspring in mice. Allergy. 2016 Jan;71(1):68–76.
63 Zou J, Chassaing B, Singh V, Pellizzon M, Ricci M, Fythe MD, et al. Fiber-Mediated Nourishment of Gut Microbiota Protects against Diet-Induced Obesity by Restoring IL-22-Mediated Colonic Health. Cell Host Microbe. 2018 Jan;23(1):41–53.e4.
64 Butel MJ. Probiotics, gut microbiota and health. Med Mal Infect. 2014 Jan;44(1):1–8.
65 Elazab N, Mendy A, Gasana J, Vieira ER, Quizon A, Forno E. Probiotic administration in early life, atopy, and asthma: a meta-analysis of clinical trials. Pediatrics. 2013 Sep; 132(3):e666–76.
66 Azad MB, Coneys JG, Kozyrskyj AL, Field CJ, Ramsey CD, Becker AB, et al. Probiotic supplementation during pregnancy or infancy for the prevention of asthma and wheeze: systematic review and meta-analysis. BMJ. 2013 Dec;347:f6471.
67 Kuperman AA, Koren O. Antibiotic use during pregnancy: how bad is it? BMC Med. 2016 Jun;14(1):91.
68 Degroote S, Hunting DJ, Baccarelli AA, Tak- ser L. Maternal gut and fetal brain connection: increased anxiety and reduced social interactions in Wistar rat offspring following peri-conceptional antibiotic exposure. Prog Neuropsychopharmacol Biol Psychiatry. 2016 Nov;71:76–82.
69 Tochitani S, Ikeno T, Ito T, Sakurai A, Yama- uchi T, Matsuzaki H. Administration of Non- Absorbable Antibiotics to Pregnant Mice to Perturb the Maternal Gut Microbiota Is Associated with Alterations in Offspring Behavior. PLoS One. 2016 Jan;11(1):e0138293.
70 Maier L, Pruteanu M, Kuhn M, Zeller G, Tel- zerow A, Anderson EE, et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018 Mar;555(7698):623–8.
71 Cussotto S, Strain CR, Fouhy F, Strain RG, Peterson VL, Clarke G, et al. Differential effects of psychotropic drugs on microbiome composition and gastrointestinal function. Psychopharmacology (Berl). 2018 Aug:1–15.
72 Csiszar K, Molnar J. Mechanism of action of tricyclic drugs on Escherichia coli and Yersinia enterocolitica plasmid maintenance and replication. Anticancer Res. 1992 Nov-Dec; 12(6B):2267–72.
73 Munoz-Bellido JL, Munoz-Criado S, Garcìa- Rodrìguez JA. Antimicrobial activity of psychotropic drugs: selective serotonin reuptake inhibitors. Int J Antimicrob Agents. 2000 Apr;14(3):177–80.
74 Olivier JD, Vallès A, van Heesch F, Afrasiab- Middelman A, Roelofs JJ, Jonkers M, et al. Fluoxetine administration to pregnant rats increases anxiety-related behavior in the off- spring. Psychopharmacology (Berl). 2011 Oct;217(3):419–32.
75 de Theije CG, Wopereis H, Ramadan M, van Eijndthoven T, Lambert J, Knol J, et al. Altered gut microbiota and activity in a murine model of autism spectrum disorders. Brain Behav Immun. 2014 Mar;37:197–206.
76 Codagnone MG, Podestá MF, Uccelli NA, Reinés A. Differential Local Connectivity and Neuroinflammation Profiles in the Medial Prefrontal Cortex and Hippocampus in the Valproic Acid Rat Model of Autism. Dev Neurosci. 2015;37(3):215–31.
77 Betts KS, Williams GM, Najman JM, Alati R. The relationship between maternal depressive, anxious, and stress symptoms during pregnancy and adult offspring behavioral and emotional problems. Depress Anxiety. 2015 Feb;32(2):82–90.
78 Zijlmans MA, Korpela K, Riksen-Walraven JM, de Vos WM, de Weerth C. Maternal pre-natal stress is associated with the infant intestinal microbiota. Psychoneuroendocrinolo- gy. 2015 Mar;53:233–45.
79 Hantsoo L, Jašarević E, Criniti S, McGeehan B, Tanes C, Sammel MD, et al. Childhood adversity impact on gut microbiota and inflammatory response to stress during pregnancy. Brain Behav Immun. 2019 Jan;75:240–50.
80 Golubeva AV, Crampton S, Desbonnet L, Edge D, O’Sullivan O, Lomasney KW, et al. Prenatal stress-induced alterations in major physiological systems correlate with gut mi- crobiota composition in adulthood. Psycho- neuroendocrinology. 2015 Oct;60:58–74.
81 Satokari R, Grönroos T, Laitinen K, Salminen S, Isolauri E. Bifidobacterium and Lactobacillus DNA in the human placenta. Lett Appl Microbiol. 2009 Jan;48(1):8–12.
82 Aagaard K, Ma J, Antony KM, Ganu R, Petro- sino J, Versalovic J. The placenta harbors a unique microbiome. Sci Transl Med. 2014 May;6(237):237ra65.
83 Jiménez E, Marín ML, Martín R, Odriozola JM, Olivares M, Xaus J, et al. Is meconium from healthy newborns actually sterile? Res Microbiol. 2008 Apr;159(3):187–93.
84 Hu J, Nomura Y, Bashir A, Fernandez-Her- nandez H, Itzkowitz S, Pei Z, et al. Diversified microbiota of meconium is affected by maternal diabetes status. PLoS One. 2013 Nov; 8(11):e78257.
85 Prince AL, Ma J, Kannan PS, Alvarez M, Gisslen T, Harris RA, et al. The placental membrane microbiome is altered among subjects with spontaneous preterm birth with and without chorioamnionitis. Am J Obstet Gynecol. 2016 May;214(5):627.e1-627.e16.
86 Perez-Muñoz ME, Arrieta MC, Ramer-Tait AE, Walter J. A critical assessment of the “sterile womb” and “in utero colonization” hypotheses: implications for research on the pioneer infant microbiome. Microbiome. 2017 Apr;5(1):48.
87 Lim ES, Rodriguez C, Holtz LR. Amniotic fluid from healthy term pregnancies does not harbor a detectable microbial community. Microbiome. 2018 May;6(1):87.
88 Biasucci G, Benenati B, Morelli L, Bessi E, Boehm G. Cesarean delivery may affect the early biodiversity of intestinal bacteria. J Nutr. 2008 Sep;138(9):1796S–800S.
89 Bäckhed F, Roswall J, Peng Y, Feng Q, Jia H, Kovatcheva-Datchary P, et al. Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell Host Microbe. 2015 May;17(5):690–703.
90 Madan JC, Hoen AG, Lundgren SN, Farzan SF, Cottingham KL, Morrison HG, et al. Association of Cesarean Delivery and Formula Supplementation With the Intestinal Microbiome of 6-Week-Old Infants. JAMA Pediatr. 2016 Mar;170(3):212–9.
91 Biasucci G, Rubini M, Riboni S, Morelli L, Bessi E, Retetangos C. Mode of delivery affects the bacterial community in the new- born gut. Early Hum Dev. 2010 Jul;86 Suppl 1:13–5.
92 Salminen S, Gibson GR, McCartney AL, Isolauri E. Influence of mode of delivery on gut microbiota composition in seven year old children. Gut. 2004 Sep;53(9):1388–9.
93 Brumbaugh DE, Arruda J, Robbins K, Ir D, Santorico SA, Robertson CE, et al. Mode of Delivery Determines Neonatal Pharyngeal Bacterial Composition and Early Intestinal Colonization. J Pediatr Gastroenterol Nutr. 2016 Sep;63(3):320–8.
94 Dogra S, Sakwinska O, Soh SE, Ngom-Bru C, Brück WM, Berger B, et al.; GUSTO Study Group. Dynamics of infant gut microbiota are influenced by delivery mode and gestational duration and are associated with subsequent adiposity. MBio. 2015 Feb;6(1):6.
95 Grześkowiak Ł, Sales Teixeira TF, Bigonha SM, Lobo G, Salminen S, Ferreira CL. Gut Bifidobacterium microbiota in one-month- old Brazilian newborns. Anaerobe. 2015 Oct;35(Pt B):54–8.
96 Martin R, Makino H, Cetinyurek Yavuz A, Ben-Amor K, Roelofs M, Ishikawa E, et al. Early-Life Events, Including Mode of Delivery and Type of Feeding, Siblings and Gender, Shape the Developing Gut Microbiota. PLoS One. 2016 Jun;11(6):e0158498.
97 Tun HM, Bridgman SL, Chari R, Field CJ, Guttman DS, Becker AB, et al.; Canadian Healthy Infant Longitudinal Development (CHILD) Study Investigators. Roles of Birth Mode and Infant Gut Microbiota in Inter-generational Transmission of Overweight and Obesity from Mother to Offspring. JAMA Pediatr. 2018 Apr;172(4):368–77.
98 Chu DM, Ma J, Prince AL, Antony KM, Seferovic MD, Aagaard KM. Maturation of the infant microbiome community structure and function across multiple body sites and in relation to mode of delivery. Nat Med. 2017 Mar;23(3):314–26.
99 Singh SB, Madan J, Coker M, Hoen A, Baker ER, Karagas MR, et al. Does birth mode modify associations of maternal pre-pregnancy BMI and gestational weight gain with the infant gut microbiome? Int J Obes. 2019 Feb.
100 McCann A, Ryan FJ, Stockdale SR, Dalmas- so M, Blake T, Ryan CA, et al. Viromes of one year old infants reveal the impact of birth mode on microbiome diversity. PeerJ. 2018 May;6:e4694.
101 Jakobsson HE, Abrahamsson TR, Jenmalm MC, Harris K, Quince C, Jernberg C, et al. Decreased gut microbiota diversity, delayed Bacteroidetes colonisation and reduced Th1 responses in infants delivered by caesarean section. Gut. 2014 Apr;63(4):559–66.
102 Azad MB, Konya T, Maughan H, Guttman DS, Field CJ, Chari RS, et al.; CHILD Study Investigators. Gut microbiota of healthy Canadian infants: profiles by mode of delivery and infant diet at 4 months. CMAJ. 2013 Mar;185(5):385–94.
103 Borre YE, O’Keeffe GW, Clarke G, Stanton C, Dinan TG, Cryan JF. Microbiota and neurodevelopmental windows: implications for brain disorders. Trends Mol Med. 2014 Sep; 20(9):509–18.
104 Bisgaard H, Li N, Bonnelykke K, Chawes BL, Skov T, Paludan-Muller G, et al. Reduced di- versity of the intestinal microbiota during infancy is associated with increased risk of allergic disease at school age. J Allergy Clin Immunol. 2011 Sep;128(3):646–52.e1–5.
105 Roduit C, Scholtens S, de Jongste JC, Wijga AH, Gerritsen J, Postma DS, et al. Asthma at 8 years of age in children born by caesarean section. Thorax. 2009 Feb;64(2):107–13.
106 Montoya-Williams D, Lemas DJ, Spiryda L, Patel K, Carney OO, Neu J, et al. The Neonatal Microbiome and Its Partial Role in Mediating the Association between Birth by Cesarean Section and Adverse Pediatric Outcomes. Neonatology. 2018;114(2):103–11.
107 Blustein J, Attina T, Liu M, Ryan AM, Cox LM, Blaser MJ, et al. Association of caesarean delivery with child adiposity from age 6 weeks to 15 years. Int J Obes. 2013 Jul;37(7): 900–6.
108 Penders J, Thijs C, Vink C, Stelma FF, Snijders B, Kummeling I, et al. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics. 2006 Aug;118(2):511–21.
109 Castillo-Ruiz A, Mosley M, Jacobs AJ, Hoffiz YC, Forger NG. Birth delivery mode alters perinatal cell death in the mouse brain. Proc Natl Acad Sci USA. 2018 Nov;115(46): 11826–31.
110 Chiesa M, Guimond D, Tyzio R, Pons-Ben- naceur A, Lozovaya N, Burnashev N, et al. Term or Preterm Cesarean Section Delivery Does Not Lead to Long-term Detrimental Consequences in Mice. Cereb Cortex. 2018 May.
111 Curran EA, Kenny LC, Dalman C, Kearney PM, Cryan JF, Dinan TG, et al. Birth by caesarean section and school performance in Swedish adolescents- a population-based study. BMC Pregnancy Childbirth. 2017 Apr;17(1):121.
112 O’Neill SM, Curran EA, Dalman C, Kenny LC, Kearney PM, Clarke G, et al. Birth by Caesarean Section and the Risk of Adult Psychosis: A Population-Based Cohort Study. Schizophr Bull. 2016 May;42(3):633–41.
113 Moya-Pérez A, Luczynski P, Renes IB, Wang S, Borre Y, Anthony Ryan C, et al. Intervention strategies for cesarean section-induced alterations in the microbiota-gut-brain axis. Nutr Rev. 2017 Apr;75(4):225–40.
114 Dominguez-Bello MG, De Jesus-Laboy KM, Shen N, Cox LM, Amir A, Gonzalez A, et al. Partial restoration of the microbiota of cesarean-born infants via vaginal microbial transfer. Nat Med. 2016 Mar;22(3):250–3.
115 Sandhu KV, Sherwin E, Schellekens H, Stan- ton C, Dinan TG, Cryan JF. Feeding the microbiota-gut-brain axis: diet, microbiome, and neuropsychiatry. Transl Res. 2017 Jan; 179:223–44.
116 Hesla HM, Stenius F, Jäderlund L, Nelson R, Engstrand L, Alm J, et al. Impact of lifestyle on the gut microbiota of healthy infants and their mothers—the ALADDIN birth cohort. FEMS Microbiol Ecol. 2014 Dec;90(3):791– 801.
117 Deoni S, Dean D 3rd, Joelson S, O’Regan J, Schneider N. Early nutrition influences developmental myelination and cognition in infants and young children. Neuroimage. 2018 Sep;178:649–59.
118 Jost T, Lacroix C, Braegger C, Chassard C. Assessment of bacterial diversity in breast milk using culture-dependent and culture- independent approaches. Br J Nutr. 2013 Oct;110(7):1253–62.
119 Cabrera-Rubio R, Collado MC, Laitinen K, Salminen S, Isolauri E, Mira A. The human milk microbiome changes over lactation and is shaped by maternal weight and mode of delivery. Am J Clin Nutr. 2012 Sep;96(3): 544–51.
120 Vandenplas Y. Oligosaccharides in infant formula. Br J Nutr. 2002 May;87(Suppl 2):S293–6.
121 Barile D, Rastall RA. Human milk and re- lated oligosaccharides as prebiotics. Curr Opin Biotechnol. 2013 Apr;24(2):214–9.
122 Garrido D, Dallas DC, Mills DA. Consump- tion of human milk glycoconjugates by infant-associated bifidobacteria: mechanisms and implications. Microbiology. 2013 Apr; 159(Pt 4):649–64.
123 Vandenplas Y, De Greef E, Veereman G. Prebiotics in infant formula. Gut Microbes. 2014;5(6):681–7.
124 Bergström A, Skov TH, Bahl MI, Roager HM, Christensen LB, Ejlerskov KT, et al. Establishment of intestinal microbiota during early life: a longitudinal, explorative study of a large cohort of Danish infants. Appl Environ Microbiol. 2014 May;80(9):2889–900.
125 Hollister EB, Riehle K, Luna RA, Weidler EM, Rubio-Gonzales M, Mistretta TA, et al. Structure and function of the healthy pre-adolescent pediatric gut microbiome. Mi- crobiome. 2015 Aug;3(1):36.
126 Grüber C, van Stuijvenberg M, Mosca F, Moro G, Chirico G, Braegger CP, et al.; MIPS 1 Working Group. Reduced occurrence of early atopic dermatitis because of immunoactive prebiotics among low-atopy-risk infants. J Allergy Clin Immunol. 2010 Oct;126(4):791–7.
127 Tarr AJ, Galley JD, Fisher SE, Chichlowski M, Berg BM, Bailey MT. The prebiotics 3’Si- alyllactose and 6’Sialyllactose diminish stressor-induced anxiety-like behavior and colonic microbiota alterations: evidence for effects on the gut-brain axis. Brain Behav Immun. 2015 Nov;50:166–77.
128 Burokas A, Arboleya S, Moloney RD, Peter- son VL, Murphy K, Clarke G, et al. Targeting the microbiota-gut-brain axis: prebiotics have anxiolytic and antidepressant-like effects and reverse the impact of chronic stress in mice. Biol Psychiatry. 2017 Oct;82(7): 472–87.
129 Korpela K, Salonen A, Virta LJ, Kumpu M, Kekkonen RA, de Vos WM. Lactobacillus rhamnosus GG intake modifies preschool children’s intestinal microbiota, alleviates penicillin-associated changes, and reduces antibiotic use. PLoS One. 2016 Apr; 11(4):e0154012.
130 Pärtty A, Kalliomäki M, Wacklin P, Salminen S, Isolauri E. A possible link between early probiotic intervention and the risk of neuropsychiatric disorders later in childhood: a randomized trial. Pediatr Res. 2015 Jun; 77(6):823–8.
131 Panigrahi P, Parida S, Nanda NC, Satpathy R, Pradhan L, Chandel DS, et al. A randomized synbiotic trial to prevent sepsis among infants in rural India. Nature. 2017 Aug; 548(7668):407–12.
132 Weizman Z, Asli G, Alsheikh A. Effect of a probiotic infant formula on infections in child care centers: comparison of two probiotic agents. Pediatrics. 2005 Jan;115(1):5–9.
133 Indrio F, Di Mauro A, Riezzo G, Civardi E, Intini C, Corvaglia L, et al. Prophylactic use of a probiotic in the prevention of colic, regurgitation, and functional constipation: a randomized clinical trial. JAMA Pediatr. 2014 Mar;168(3):228–33.
134 Parracho HM, Gibson GR, Knott F, Bosscher D, Kleerebezem M, McCartney AL. A double-blind, placebo-controlled, cross- over-designed probiotic feeding study in children diagnosed with autistic spectrum disorders. Int J Probiotics Prebiotics. 2010; 5(2):69–74.
135 Gareau MG, Jury J, MacQueen G, Sherman PM, Perdue MH. Probiotic treatment of rat pups normalises corticosterone release and ameliorates colonic dysfunction induced by maternal separation. Gut. 2007 Nov;56(11): 1522–8.
136 Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013 Dec;155(7):1451–63.
137 Callaghan BL, Cowan CS, Richardson R. Treating Generational Stress: Effect of Paternal Stress on Development of Memory and Extinction in Offspring Is Reversed by Probiotic Treatment. Psychol Sci. 2016 Sep; 27(9):1171–80.
138 Cowan CS, Callaghan BL, Richardson R. The effects of a probiotic formulation (Lactoba-cillus rhamnosus and L. helveticus) on developmental trajectories of emotional learning in stressed infant rats. Transl Psychiatry. 2016 May;6(5):e823.
139 Desbonnet L, Garrett L, Clarke G, Kiely B, Cryan JF, Dinan TG. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience. 2010 Nov;170(4):1179–88.
140 Yassour M, Vatanen T, Siljander H, Hämäläinen AM, Härkönen T, Ryhänen SJ, et al.; DIABIMMUNE Study Group. Natural history of the infant gut microbiome and impact of antibiotic treatment on bacterial strain diversity and stability. Sci Transl Med. 2016 Jun;8(343):343ra81.
141 Leclercq S, Mian FM, Stanisz AM, Bindels LB, Cambier E, Ben-Amram H, et al. Low- dose penicillin in early life induces long-term changes in murine gut microbiota, brain cytokines and behavior. Nat Com- mun. 2017 Apr;8:15062.
142 O’Mahony SM, Felice VD, Nally K, Savignac HM, Claesson MJ, Scully P, et al. Disturbance of the gut microbiota in early-life selectively affects visceral pain in adulthood without impacting cognitive or anxiety-related behaviors in male rats. Neuroscience. 2014 Sep;277:885–901.
143 Parracho HM, Bingham MO, Gibson GR, McCartney AL. Differences between the gut microflora of children with autistic spectrum disorders and that of healthy children. J Med Microbiol. 2005 Oct;54(Pt 10):987– 91.
144 Finegold SM, Dowd SE, Gontcharova V, Liu C, Henley KE, Wolcott RD, et al. Pyrose- quencing study of fecal microflora of autistic and control children. Anaerobe. 2010 Aug; 16(4):444–53.
145 Wang L, Conlon MA, Christophersen CT, Sorich MJ, Angley MT. Gastrointestinal microbiota and metabolite biomarkers in children with autism spectrum disorders. Bio- markers Med. 2014;8(3):331–44.
146 Finegold SM. Desulfovibrio species are potentially important in regressive autism. Med Hypotheses. 2011 Aug;77(2):270–4.
147 Kang DW, Ilhan ZE, Isern NG, Hoyt DW, Howsmon DP, Shaffer M, et al. Differences in fecal microbial metabolites and microbiota of children with autism spectrum disorders. Anaerobe. 2018 Feb;49:121–31.
148 Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001 Jun;49(12): 1023–39.
149 O’Mahony SM, Marchesi JR, Scully P, Cod- ling C, Ceolho AM, Quigley EM, et al. Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biol Psychiatry. 2009 Feb;65(3):263–7.
150 Bailey MT, Coe CL. Maternal separation dis- rupts the integrity of the intestinal microflora in infant rhesus monkeys. Dev Psychobi- ol. 1999 Sep;35(2):146–55.
151 McVey Neufeld KA, O’Mahony SM, Hoban AE, Waworuntu RV, Berg BM, Dinan TG, et al. Neurobehavioural effects of Lactobacillus rhamnosus GG alone and in combination with prebiotics polydextrose and galactooli-gosaccharide in male rats exposed to early- life stress. Nutr Neurosci. 2017 Nov:1–10.
152 Marlow G, Ellett S, Ferguson IR, Zhu S, Karunasinghe N, Jesuthasan AC, et al. Tran- scriptomics to study the effect of a Mediterranean-inspired diet on inflammation in Crohn's disease patients. Hum Genomics. 2013 Nov;7:24.
153 Bindels LB, Delzenne NM, Cani PD, Walter J. Towards a more comprehensive concept for prebiotics. Nat Rev Gastroenterol Hepatol. 2015 May;12(5):303–10.
154 Hemarajata P, Versalovic J. Effects of probiotics on gut microbiota: mechanisms of intestinal immunomodulation and neuro-modulation. Therap Adv Gastroenterol. 2013 Jan;6(1):39–51.

