Microbiota and Human Milk Oligosaccharides in Premature Infants
Author: Jean-Michel Hascoët
Key Messages:
- The gut microbiota of infants may show different patterns associated with maturity and outcome that can be improved by prebiotics such as HMO.
- Preterm infants have a risk of specific HMO deficiencies leading to poorer outcome.
- HMO supplementation given shortly after birth is well tolerated with a trend towards better feeding tolerance, and supports early postnatal growth
Preterm birth is an important risk factor for neonatal sepsis and other comorbidities, such as appropriate growth that may be challenging.1 Gut microbiota plays a significant role in infant health. Breastfed term infants have a gut microbiota dominated by bifidobacteria that is associated with reduced infection rates, while the microbiota of formula-fed infants is more heterogeneous.2 An observational study of 577 very preterm newborns showed that gut microbiota was not dominated by bifidobacteria in early life, but was related to infant maturity and indeed associated with the outcome.3 In addition, because of the high risk of early onset infection, probabilistic antibiotic usage is frequent in premature infants and causes a further imbalance in gut microbiota.4 Prebiotics may promote the colonization of beneficial microbiota and prevent antibiotic associated side effects.5 Human milk oligosaccharides (HMOs) are a type of prebiotic representing the third largest solid component in human milk after lactose and lipids, and impact on the infant gastrointestinal microbiota.6
An existent gap
Preterm infants have a risk of specific HMO deficiencies. In a study of 500 milk samples from 25 mothers breastfeeding very preterm infants, versus 28 mothers of term infants, the concentrations of a number of HMOs were significantly lower in preterm compared to term milk.7 In addition, a study in 30 German women showed that there might be a genetic variation of HMO secretor profiles. This study suggests that milk of non-secretor women exerts an impaired biological protection versus milks of secretors.8
Likewise, in a study of 410 very premature infants, low secretor phenotype was significantly associated with necrotizing enterocolitis incidence, and non-secretor genotype was associated with gram-negative sepsis.9 Thus, the question arises: could HMO supplementation of microbiota benefit premature infants?
HMOs supplementation in premature infants10 A multicenter randomized controlled intervention study was undertaken to assess the effect of a supplement containing 2 specific HMOs on very preterm infants. The primary objective was to demonstrate feeding tolerance measured by non-inferiority in days to reach full enteral feeding (FEF). Secondary outcomes included growth parameters, gastro-intestinal symptoms and adverse events up to 12 months corrected age. Administration of the products occurred after 24 hours of trophic feeding and within the first 5 days postnatal age (FIG. 1)
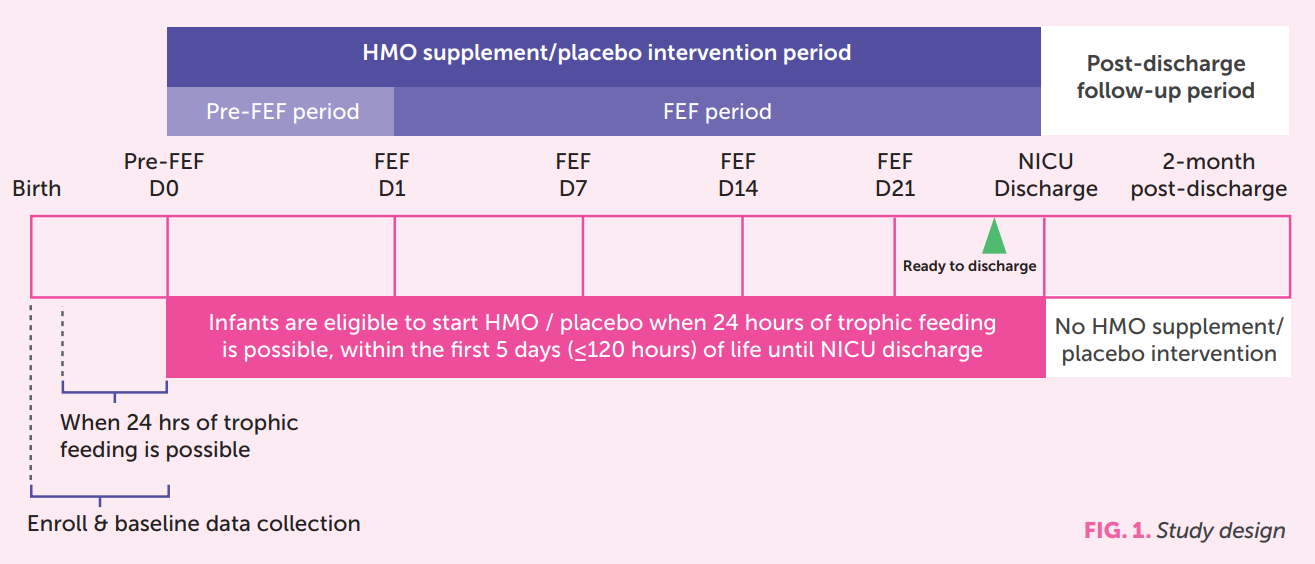
43 infants were included in each group. Gestational age was (Mean ± Standard deviation) 29.7±1.4 vs. 30.2±1.4 weeks. Both groups were comparable for weight, length, and head circumference for age z-scores at birth. The supplement was found to be safe, well-tolerated, and associated with a reduction in time to reach FEF as well as improved growth patterns in length and head circumference at discharge (FIG. 2).
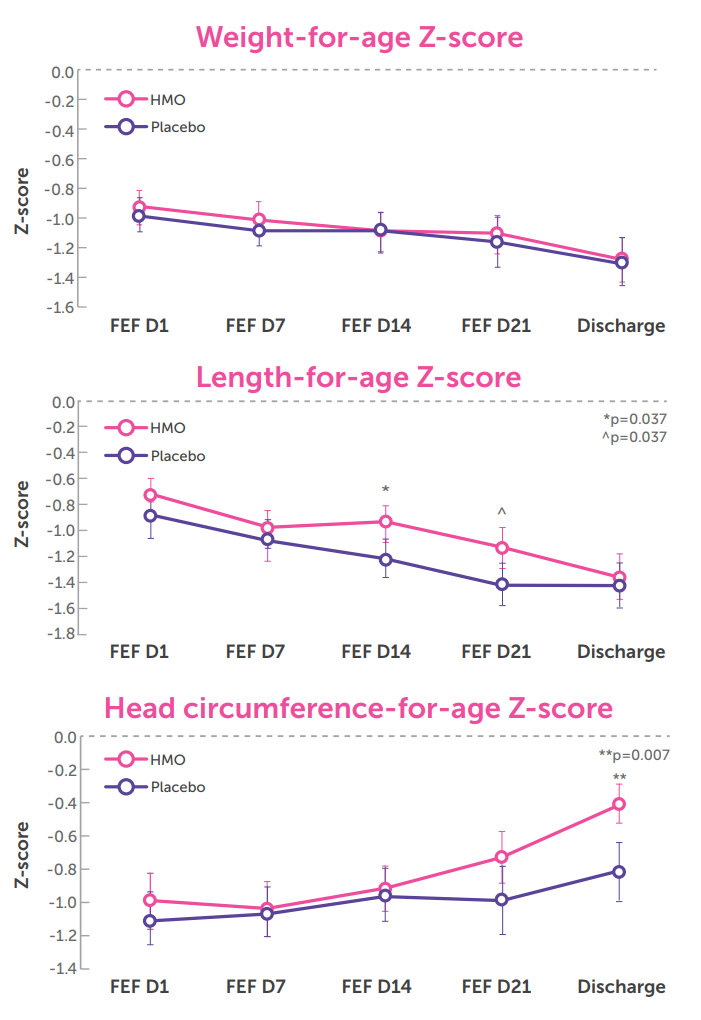
In conclusion, the gut microbiota of premature infants may show different patterns associated with maturity and low levels of HMOs leading to poorer outcomes. HMO supplementation given shortly after birth is well tolerated with a trend towards better feeding tolerance, and supports early postnatal growth, which may have a positive impact on developmental outcomes.
References
- Institute of Medicine (US) Committee on Understanding Premature Birth and Assuring Health Outcomes. Mortality and Acute Complications in Preterm Infants. In. Preterm Birth: Causes, Consequences, and Prevention. Washington, DC: National Academies Press; 2007.
- Harmsen HJM, Wildeboer–Veloo ACM, Raangs GC, Wagendorp AA, Klijn N, Bindels JG, et al. Analysis of Intestinal Flora Development in Breast-Fed and Formula-Fed Infants by Using Molecular Identification and Detection Methods: J Pediatr Gastroenterol Nutr. janv 2000;30(1):61-7.
- Rozé J-C, Ancel P-Y, Marchand-Martin L, Rousseau C, Montassier E, Monot C, et al. Assessment of Neonatal Intensive Care Unit Practices and Preterm Newborn Gut Microbiota and 2-Year Neurodevelopmental Outcomes. JAMA Netw Open. 23 sept 2020;3(9):e2018119.
- Greenwood C, Morrow AL, Lagomarcino AJ, et al. Early empiric antibiotic use in preterm infants is associated with lower bacterial diversity and higher relative abundance of Enterobacter. J Pediatr. 2014;165(1):23-29.
- Novak J, Katz JA. Probiotics and prebiotics for gastrointestinal infections. Curr Infect Dis Rep. 2006 Mar;8(2):103-9. doi: 10.1007/s11908-006- 0005-9.
- Zivkovic AM, German JB, Lebrilla CB, Mills DA. Human milk glycobiome and its impact on the infant gastrointestinal microbiota. Proc Natl Acad Sci U S A. 2011;108 Suppl 1(Suppl 1):4653-4658.
- Austin S, De Castro CA, Sprenger N, Binia A, Affolter M, Garcia-Rodenas CL, et al. Human Milk Oligosaccharides in the Milk of Mothers Delivering Term versus Preterm Infants. Nutrients. 5 juin 2019;11(6):1282.
- Thurl S, Munzert M, Henker J, Boehm G, Müller-Werner B, Jelinek J, Stahl B. Variation of human milk oligosaccharides in relation to milk groups and lactational periods. Br J Nutr. 2010 Nov;104(9):1261-71. doi: 10.1017/ S0007114510002072.
- Morrow AL, Meinzen-Derr J, Huang P, Schibler KR, Cahill T, Keddache M, Kallapur SG, Newburg DS, Tabangin M, Warner BB, Jiang X. Fucosyltransferase 2 non-secretor and low secretor status predicts severe outcomes in premature infants. J Pediatr. 2011 May;158(5):745-51. doi: 10.1016/j. jpeds.2010.10.043.
- Hascoët JM, Chevallier M, Gire C, Brat R, Rozé JC, Norbert K, Chen Y, Hartweg M, Billeaud C. Use of a Liquid Supplement Containing 2 Human Milk Oligosaccharides: The First Double-Blind, Randomized, Controlled Trial in Pre-term Infants. Front Pediatr. 2022 Apr 25;10:858380. doi: 10.3389/fped.2022.858380
