Metabolomics in Human Milk Research
Abstract
The link between food and health is complex, particularly for the developing neonate, as the period after birth is the time when long-term programming is occurring notably in the neurologic, immune, and metabolic regulatory systems. Breastfeeding is known to have short- and long-term benefits, and yet the intricate relationship of this unique food with the neonate is not fully understood. Application of multiomic approaches incorporating new bioinformatic tools will allow for better characterization of phenotypes over the traditional approaches that were limited to crude assessment of growth parameters and observation of clinical disease. Metabolomics has the capability of allowing for a relatively noninvasive assessment of phenotypes via the assessment of small molecules in biofluids such as serum or urine that provides an opportunity to assess metabolism systemically in the developing neonate. Metabolomics can also be used to assess the metabolic activities of gut microbes through measurement of microbial by-products in the stool. Understanding the composition of human milk, how its components work synergistically together, and how they change over time will provide insight into how immunity and metabolism is established in early life, and how it can potentially prevent the development of chronic diseases in later life.
Introduction
The postnatal period for human infants is recognized as a key period for growth and development, particularly for the rapidly developing brain. All of the infant’s metabolic, immunologic, intestinal, and physiologic systems are also rapidly changing. The ability of milk to nourish, fuel, and supply substrates to this growth has been recognized [1]. However, the role of milk in orchestrating the entire metabolic process is not clear. A new generation of tools are now available to interrogate both milk and its effects on infant metabolism. Here, metabolomics is defined as the science of analyzing metabolites as an ensemble with sufficient diversity to map those metabolites onto pathways and sufficient quantitative accuracy to estimate metabolite flows through those pathways.
Brain growth during infancy has been positively associated with intelligence [2, 3], and human milk helps promote that development, particularly white matter development [4]. Indeed, the human brain volume increases between 8 and 15% every 3 months from birth until approximately 18 months of age. Brain metabolites also change with dramatic increases in N-acetylaspartate, creatine, and glutamate and decreases in myoinositol in the cerebral white matter and cortex during the first 3 postnatal months [5]. Epidemiological evidence suggests that breastfeeding practice is positively associated with intelligence and educational attainment at age 30 [6], and negatively associated with obesity, cardiovascular disease [7], and diabetes [8] in adulthood. How human milk is able to fuel and shape metabolism and guide overall health and development is an ongoing area of active research, and clues are beginning to emerge that the microbiota within the infant gastrointestinal (GI) tract are important.
The period immediately after birth, and for the first 2–3 years of the child’s life, is a key period for the development of intestinal microbiota [reviewed in 9, 10]. In the GI tract resides an ecosystem where microbes must compete in order to survive and persist, and the host must shape the microbiota in order to foster a beneficial community [11]. One theory states that for symbiont-directed control, where a microbe alters global host phenotype to increase its own fitness, there must be low microbial diversity and limited competition between microbes [11]. Interestingly, until the weaning period, breastfed infants have lower microbial diversity than formula-fed infants [reviewed in 12]. Infancy is also a time of instability with respect to the gut microbiota as evidenced by greater inter individual variability compared with adults [reviewed in 13]. This instability represents an important window of opportunity for the development of the gut microbial community. A recent study using gnotobiotic mice demonstrated that there is a critical window of time for intestinal immune development, and if conventionalization does not occur during that period, immune development can- not be fully achieved [14]. Evidence is accumulating that the immune system is linked inextricably with global metabolism [15]. Thus, it stands to reason that immune development will have important consequences for metabolic development [16], and if both are not optimally established early in life, consequences may be realized later in life.
The complex nature of milk therefore means that it has important functions for optimizing the health and the microbiota of the infant. Understanding the intricacies of the system at each stage of childhood that are vital for establishing the eventual host phenotype, including immunity, metabolism, and brain health, will be important for understanding the role of milk and its components in shaping health. While we are not yet at a stage where these complex relationships have been elucidated, linking host genetics and microbial ecology with the environment, including diet (macro- and micronutrient composition), and phenotype (measured through metabolomic analysis of serum, urine, and feces) will help us move toward that goal.
Human Milk and the Human Milk Metabolome
During the first few months of life, human milk provides essential nutrients for the infant while helping to establish the bacterial species that will constitute the gut microbiome. In addition to providing the essentials for growth and development, proteins, fatty acids, carbohydrates, and micronutrients, human milk provides a variety of cytokines, inflammatory mediators, and signaling molecules [reviewed in 17, 18].
Human milk varies greatly throughout lactation, with the colostrum very rich with immune factors and oligosaccharides that tend to decrease as lactation progresses [reviewed in 17]. Interestingly, we and others [19–23] observed a number of metabolites that change in concentration over time in human milk that includes amino acids, sugars, fatty acids, and others (Table 1). In general, oligosaccharides tend to decrease over time, while increases in lactose, several amino acids, as well as short- and medium-chain free fatty acids are noted. What this means is that milk maturation is not stochastic. Its composition is carefully controlled by the mammary gland that is guided by maternal genetics, continuously changing to meet the nutrient requirements of the neonate and help guide microbial succession throughout the period of exclusive milk feeding and possibly beyond. It is interesting to point out that microbial and fecal metabolic profiles are more similar between formula-fed infants from different mothers than breastfed infants from different mothers [24, 25], which could be attributed to the differences in milk composition between mothers who are breastfeeding and driven by changes in milk composition over the lactation period. In contrast, the composition of formula does not change. These results imply that diet (breast- or formula feeding) is a key driver in selecting microbes to colonize the GI tract.
One example of how maternal genetics tightly controls milk composition may be observed through the correlation of 2 of the more abundant oligosaccharides: 2′-fucosyllactose (2′FL) and 3-fucosyllactose (3FL). The concentration of these two oligosaccharides is dictated by the expression levels of enzymes encoded by the fucosyltransferase 2 (FUT2) and fucosyltransferase 3 (FUT3) genes, both of which are located on chromosome 19 [19, 26]. Production of 2′FL or 3FL is highly correlated such that as the concentration of 2′FL increases, 3FL decreases (Pearson’s correlation r = –0.76; Fig. 1a). In women who are secretors (having a functional FUT2 gene), during the course of the first 3 months of lactation 2′FL decreases from approximately 8 mmol/L in colostrum to 3 mmol/L, whereas 3FL increases from approximately 0.3 mmol/L in colostrum to 1.6 mmol/L, where the concentrations of each oligosaccharide begin to level out [19, 20] (Fig. 1b). The reason for this correlated change in concentration is not fully understood. However, human milk oligosaccharides have been credited with developing the microbiota, and in particular selecting for and maintaining high levels of Bifidobacterium longum subsp. infantis (B. infantis) in the infant GI tract during the period of exclusive human milk feeding, in addition to helping to build the immune system [9, 10]. These 2 oligosaccharides have also been recognized as having antiviral properties through acting as decoys to prevent binding of viruses to the GI tract [27, 28]. Human milk oligosaccharides have been reported to be absorbed into the blood, and 2′FL has specifically been shown to decrease plasmacytokine levels [29]. Additionally, 2′FL has been shown to modulate CD14 expression on human enterocytes to attenuate LPS-induced inflammation [30]. Controlling systemic inflammation [25] may be particularly important in the first few months of life, as circulating levels of cytokines such as IL-6 may modulate fatty acid metabolism and induce insulin resistance [reviewed in 16], which could have important consequences on metabolic development.
Another interesting metabolite that increases over time in human milk is urea. We observed that it increases from 2.7 mmol/L in colostrum to 4.5 mmol/L on postpartum day 90 [19, 20]. This increase may be a way to help create a steady nitrogen source for the growing populations of microbes in the infant GI tract [19]. While most of the free amino acids are low in concentration in human milk throughout lactation (<1 mmol/L), several do increase over time (Table 1). In particular, glutamate increases from 0.5 mmol/L in colostrum to 1.5 mmol/L in mature milk over the first 3 months [19, 20]. Dietary glutamate is extensively metabolized by the intestinal epithelium [reviewed in 31], and this increase may be important for maturation of the intestinal tract of infants; however, more re- search needs to be done to fully understand the role of milk-derived free glutamate in the developing neonate.
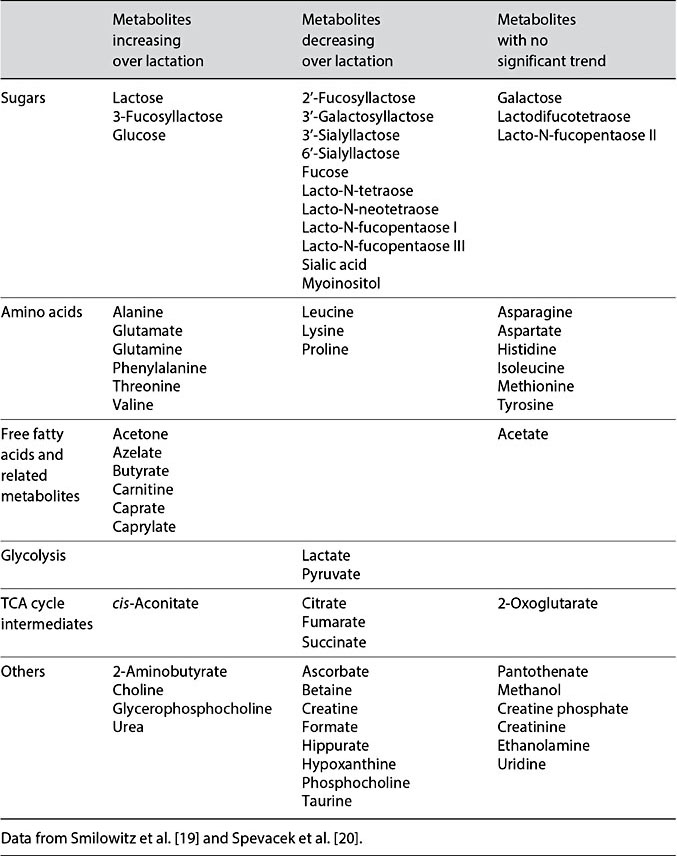
Table 1. Variation in milk metabolites over time in women who are secretors
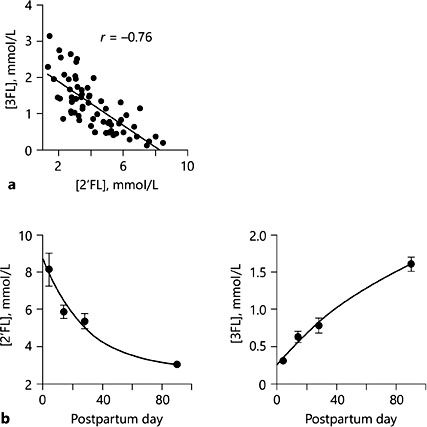
Fig. 1. a Pearson correlation of 2′- fucosyllactose (2′FL) with 3FL. b Changes in the con- centration (mmol/L) of 2′FL and 3FL from early lactation to postpartum day 90. Data from Smilowitz et al. [19] and Spevacek et al. [20].
Human Milk and Infant Health
The impact of human milk on infant metabolism can be demonstrated through comparison of the metabolomes of infants that have been formula fed and breastfed. Breastfed infants have been reported to have higher total cholesterol and LDL-C than formula-fed infants [32]. Additionally, breastfed infants have lower levels of short-chain unsaturated and higher levels of longer-chain poly- unsaturated fatty acids containing phosphatidylcholines [33]. In contrast, breastfed infants have lower levels of polyunsaturated fatty acid-containing long-chain triglycerides, and higher levels of shorter-chain sphingomyelin and 16:0 and 20:4 cholesterol esters than formula-fed infants [33]. Breastfed infants also have higher fasting levels of acetate, acetone, myoinositol, glutamine, proline, and formate, as well as lower levels of urea, creatine, essential amino acids, and their by-products (threonine and valine, 2-hydroxybutyrate and 3-hydroxy- isobutyrate), choline, and dimethyl sulfone than formula-fed infants (Table 2) [34]. In the postprandial state, breastfed infants have higher acetate, acetone, myoinositol, formate, methanol, and betaine, and lower 2-hydroxybutyrate, 3-hydroxyisobutyrate, alanine, isoleucine, leucine, lysine, methionine, proline, threonine, tyrosine, valine, choline, creatine, dimethyl sulfone, and urea (Table 3) [34]. Similar differences were observed in nonhuman primates [25].
Analysis of the urine metabolome of formula- and breastfed infants largely mirrors what is observed in the serum metabolome, with differences in metabolites related to protein and amino-acid metabolism, ketogenesis, and fatty-acid oxidation [35]. The urine metabolome also reveals differences in gut microbial function, as was observed with lower TMAO (trimethylamine-N-oxide) in breastfed compared with formula-fed infants [25].
Analysis of the fecal metabolome can reveal functionality of the microbes within the GI tract of infants. For example, fecal metabolome analysis of breast- fed infants revealed evidence of lower protein fermentation [36] and lower levels of short-chain fatty acids (propionate, butyrate, and acetate) and free amino acids, as well as higher levels of lactate and fucosylated oligosaccharides compared with formula-fed infants [35, 37, 38]. Measurement of fucosylated oligosaccharides in the stool is directly related to the consumption of human milk, as bovine milk (which the majority of formulas are based from) does not contain fucosylated oligosaccharides. Differences in lactate and short-chain fatty acids between breastfed and formula-fed infants can also be correlated with the resident microbes in the GI tract of breastfed infants. Compared with formula-fed infants, breastfed infants have higher levels bacteria from the Bifidobacterium and Lactobacillus genera, which produce lactate and acetate as primary fermentation products [10]. Acetate is higher in the plasma of breastfed infants (Tables 2, 3), which may suggest that acetate produced by these bacteria may be absorbed. Evidence of temporal changes in the fecal metabolome has also been reported [24], which implies important changes in microbial structure and function during development.
Although there are common metabolome differences reflected in the blood, urine, and feces between breastfed and formula-fed infants, it is important to note that the composition of formulas vary depending on the study. Indeed, formulas on the market vary widely with differences in macro- and micronutrient composition. Changing one component in the formula can result in profound changes to an infant’s metabolism, the structure and function of the gut microbiome, or both. For instance, a recent study revealed that replacing lactose in infant formula with corn-syrup solids resulted in a lowering of many amino ac- ids measured in the postprandial state (1–2 h) compared with infants fed lactose-based formula [34]. While this seems to be desirable, 2 h after feeding, glucose, ketones, and nonesterified fatty acids were lower in the infants fed formula with corn-syrup solids compared with those fed lactose-based formula, and their insulin levels were significantly higher than in breastfed infants.
Other studies have looked at adding probiotics to infant formula [24, 39, 40]. For example, supplementation of nonhuman primates with a formula containing B. animalis subsp. lactis resulted in slightly increased serum BCAA, and a structuring of the microbiota that was different from the formula-fed infants at 3 months [24]. Of potential concern was the marked increase in the fecal polyamines cadavarine and putrescine in the third month. Supplementation of human infants with a formula containing Bifidobacterium. bifidum, B. breve, B. longum, and B. infantis resulted in changes in both the structure and function (assessed through the fecal metabolome) of colonic bacteria during the period of supplementation but had no detectable long-term effects [39]. Another study where infant formula was supplemented with a probiotic/prebiotic combination of B. animalis subsp. lactis CNCM I-3446 and bovine milk oligosaccharides revealed an α diversity that was similar to breastfed infants over the course of supplementation, in addition to higher levels of the Bifidobacterium genus in general compared with control formula [40], but no metabolome changes were measured and reported. The fact that changes in metabolic parameters were observed in 2 of these studies suggests that these early gut microbes impact metabolic development of the infant and further that a metabolic assessment should be considered an important outcome when evaluating changes in formula ingredients.
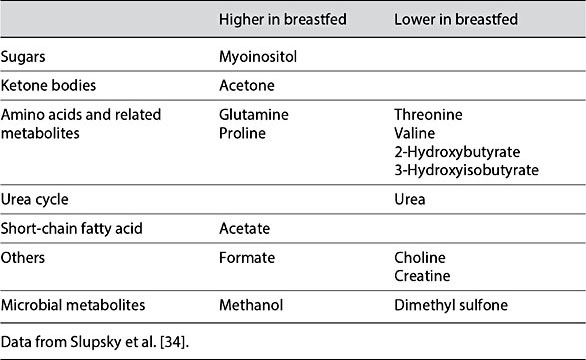
Table 2. Semifasted serum metabolite differences between breastfed and formula-fed infants
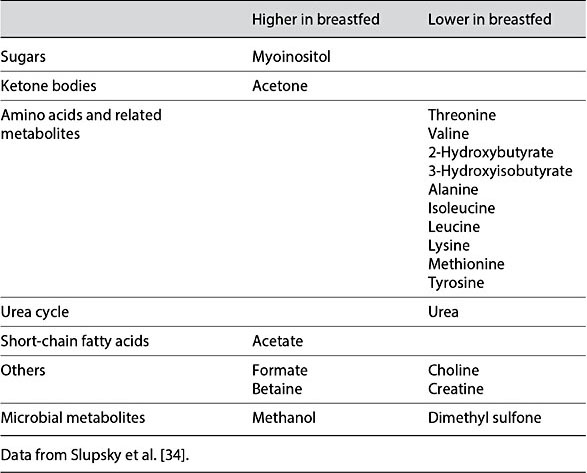
Table 3. Postprandial serum metabolite differences between breastfed and formula-fed infants
The Collaboration between Milk and the Developing Neonate
The components of human milk are unique and are specific to the growing neonate. Replacing human milk with milk from other animals has profound consequences for the developing neonate. For instance, the consistently observed in- creases in plasma amino acids in formula-fed infants may inhibit several early steps in the insulin signaling cascade [41], and the sustained increases could contribute to hepatic mitochondrial dysfunction that may be associated with future increased BMI, insulin resistance, and dyslipidemia [42].
Components of human milk may also interact directly with specific metabolic pathways, such as the mechanistic target of rapamycin (mTOR) pathway [43], to optimize development. mTOR complex 1 (mTORC1) is a nutrient-sensitive kinase that plays an important role in many aspects of cell growth, protein and lipid synthesis, as well as lipid accumulation and adipogenesis. It is particularly important in child development for the control of growth and metabolism of bone, skeletal muscle, the central nervous system, the GI tract, blood cells, and other organs [reviewed in 44]. Amino acids such as leucine can regulate mTORC1, and a correlation between the amount of leucine in the whey fraction and serum leucine levels in infants has been reported [45]. Bacteria, such as Lactobacillus plantarum, have also been shown to act on the TOR-dependent host nutrient-sensing system in Drosophila [46]. Interestingly, it was recently ob- served that L. plantarum might be vertically transmitted from mother to infant through breastfeeding [47]. Thus, there may be a connection between the types of microbes colonizing the infant GI tract and expression of the mTOR pathway, although more studies are needed to confirm.
Conclusions
Diet is remarkable in both its ability to shape gut microbiota as well as metabolism and the immune system. The exquisite linkage of microbiota, host immunity, and host metabolism hints at the complexity of human milk, and how it has been optimized for neonatal development. The changes in human milk composition throughout lactation (increases and decreases in many metabolites) likely reflect the changing needs of the neonate and the gut microbiota. Analysis of the metabolome (either serum or urine or both) reflects the human phenotype and should be considered an essential component of any study that aims to capture the metabolic effects of diet. Analysis of the fecal metabolome can inform on the function of the gut microbes. Once a healthy baseline has been defined, the response to diet can be assessed through analysis of the serum, urine, and fecal metabolomes both in the short term and long term.
More studies on human milk should be done to assess how secretor status and Lewis blood type (both in the mother and the infant) affects infant development including immunity and metabolism, as well as other factors such as maternal health and environmental exposures. Incorporating additional data including genetic and epigenetic data will be important to understand individual responses to diet and microbial succession. Incorporating a nutrigenomic approach together with an analysis of microbial structure and function will also help us understand, on a deeper level, how human milk and its components affect gene expression either by themselves or through the gut microbiota. This approach will take many years but may ultimately allow us to understand the interplay between food, gut microbiota, and metabolism, and overall provide a better understanding on how to achieve optimal health through diet.
References
-
1 Hinde K, German JB: Food in an evolutionary context: insights from mother’s milk. J Sci FoodAgric 2012;92:2219–2223.
-
2 Gale CR, O’Callaghan FJ, Bredow M, et al: The influence of head growth in fetal life, in- fancy, and childhood on intelligence at the ages of 4 and 8 years. Pediatrics 2006;118: 1486–1492.
-
3 Gale CR, O’Callaghan FJ, Godfrey KM, et al: Critical periods of brain growth and cognitive function in children. Brain 2004;127:321–329.
-
4 Isaacs EB, Fischl BR, Quinn BT, et al: Impact of breast milk on intelligence quotient, brain size, and white matter development. Pediatr Res 2010;67:357–362.
-
5 Blüml S, Wisnowski JL, Nelson MD, et al: Metabolic maturation of the human brain from birth through adolescence: insights from in vivo magnetic resonance spectrosco- py. Cereb Cortex 2013;23:2944–2955.
-
6 Victora CG, Horta BL, Loret de Mola C, et al: Association between breastfeeding and intelligence, educational attainment, and income at 30 years of age: a prospective birth cohort study from Brazil. Lancet Glob Health 2015; 3:e199–e205.
-
7 Parikh NI, Hwang S-J, Ingelsson E, et al: Breastfeeding in infancy and adult cardiovascular disease risk factors. Am J Med 2009; 122:656–663.e1.
-
8 Horta BL, Loret de Mola C, Victora CG: Long-term consequences of breastfeeding on cholesterol, obesity, systolic blood pressure and type 2 diabetes: a systematic review and meta-analysis. Acta Paediatr 2015;104:30–37.
-
9 Smilowitz JT, Lebrilla CB, Mills DA, et al: Breast milk oligosaccharides: structure-function relationships in the neonate. Annu Rev Nutr 2014;34:143–169.
-
10 Underwood MA, German JB, Lebrilla CB, et al: Bifidobacterium longum subspecies infantis: champion colonizer of the infant gut. Pe- diatrRes 2015;77:229–235.
-
11 Foster KR, Schluter J, Coyte KZ, et al: The evolution of the host microbiome as an eco-system on a leash. Nature 2017;548:43–51.
-
12 Mueller NT, Bakacs E, Combellick J, et al: The infant microbiome development: mom matters. Trends Mol Med 2015;21:109–117.
-
13 Arrieta M-C, Stiemsma LT, Amenyogbe N, et al: The intestinal microbiome in early life: health and disease. Front Immunol 2014;5: 427.
-
14 El Aidy S, Hooiveld G, Tremaroli V, et al: The gut microbiota and mucosal homeostasis. Gut Microbes 2014;4:118–124.
-
15 Man K, Kutyavin VI, Chawla A: Tissue immunometabolism: development, physiology, and pathobiology. Cell Metab 2017;25:11–26.
-
16 Buck MD, Sowell RT, Kaech SM, et al: Metabolic instruction of immunity. Cell 2017;169: 570–586.
-
17 Bardanzellu F, Fanos V, Reali A: “Omics” in human colostrum and mature milk: looking to old data with new eyes. Nutrients 2017;9: 843.
-
18 Munblit D, Peroni DG, Boix-Amorós A, et al: Human milk and allergic diseases: an unsolved puzzle. Nutrients 2017;9:894.
-
19 Smilowitz JT, O’Sullivan A, Barile D, et al: The human milk metabolome reveals diverse oligosaccharide profiles. J Nutr 2013;143: 1709–1718.
-
20 Spevacek AR, Smilowitz JT, Chin EL, et al: Infant maturity at birth reveals minor differences in the maternal milk metabolome in the first month of lactation. J Nutr 2015;145: 1698–1708.
-
21 Sundekilde UK, Downey E, O’Mahony JA, et al: The effect of gestational and lactational age on the human milk metabolome. Nutrients 2016;8:E304.
-
22 Wu J, Domellöf M, Zivkovic AM, et al: NMR- based metabolite profiling of human milk: a pilot study of methods for investigating com- positional changes during lactation. Biochem Biophys Res Commun 2016;469:626–632.
-
23 Xu G, Davis JC, Goonatilleke E, et al: Absolute quantitation of human milk oligosaccha- rides reveals phenotypic variations during lactation. J Nutr 2017;147:117–124.
-
24 He X, Slupsky CM, Dekker JW, et al: Inte- grated role of Bifidobacterium animalis sub- sp. lactis supplementation in gut microbiota, immunity, and metabolism of infant rhesus monkeys.mSystems 2016;1:e00128-16.
-
25 O’Sullivan A, He X, McNiven EMS, et al: Ear- ly diet impacts infant rhesus gut microbiome, immunity, and metabolism. J Proteome Res 2013;12:2833–2845.
-
26 Austin S, De Castro CA, Bénet T, et al: Tem- poral change of the content of 10 oligosaccharides in the milk of Chinese urban mothers. Nutrients 2016;8:E346.
-
27 Koromyslova A, Tripathi S, Morozov V, et al: Human norovirus inhibition by a humanmilk oligosaccharide. Virology 2017;508:81– 89.
-
28 Weichert S, Koromyslova A, Singh BK, et al: Structural basis for norovirus inhibition by human milk oligosaccharides. J Virol 2016; 90:4843–4848.
-
29 Goehring KC, Marriage BJ, Oliver JS, et al: Similar to those who are breastfed, infants fed a formula containing 2′-fucosyllactose have lower inflammatory cytokines in a randomized controlled trial. J Nutr 2016;146:2559– 2566.
-
30 He Y, Liu S, Kling DE, et al: The human milk oligosaccharide 2′fucosyllactose modulates CD14 expression in human enterocytes, thereby attenuating LPS-induced inflammation. Gut 2016;65:33–46.
-
31 Burrin DG, Stoll B: Metabolic fate and function of dietary glutamate in the gut. Am J ClinNutr 2009;90:850S–856S.
-
32 Harit D, Faridi MMA, Aggarwal A, et al: Lip- id profile of term infants on exclusive breast- feeding and mixed feeding: a comparative study. Eur J Clin Nutr 2008;62:203–209.
-
33 Prentice P, Koulman A, Matthews L, et al: Lipidomic analyses, breast- and formula- feeding, and growth in infants. J Pediatr 2015; 166:276–281.e6.
-
34 Slupsky CM, He X, Hernell O, et al: Post- prandial metabolic response of breast-fed infants and infants fed lactose-free vs regular infant formula: a randomized controlled trial. Sci Rep 2017;7:3640.
-
35 Martin F-PJ, Moco S, Montoliu I, et al: Impact of breast-feeding and high- and low-protein formula on the metabolism and growth of infants from overweight and obese mothers. Pediatr Res 2013;75:535–543.
-
36 Chow J, Panasevich MR, Alexander D, et al: Fecal metabolomics of healthy breast-fed versus formula-fed infants before and during in vitro batch culture fermentation. J Proteome Res 2014;13:2534–2542.
-
37 Bridgman SL, Azad MB, Field CJ, et al: Fecal short-chain fatty acid variations by breast- feeding status in infants at 4 months: differences in relative versus absolute concentrations. Front. Nutr 2017;4:11.
-
38 Underwood MA, Gaerlan S, De Leoz MLA, et al: Human milk oligosaccharides in premature infants: absorption, excretion, and influence on the intestinal microbiota. Pediatr Res 2015;78:670–677.
-
39 Bazanella M, Maier TV, Clavel T, et al: Ran- domized controlled trial on the impact of early-life intervention with bifidobacteria on the healthy infant fecal microbiota and metabolome. Am J Clin Nutr 2017;106:1274– 1286.
-
40Simeoni U, Berger B, Junick J, et al: Gut microbiota analysis reveals a marked shift to bifidobacteria by a starter infant formula containing a synbiotic of bovine milk-derived oligosaccharides and Bifidobacterium animalis subsp. lactis CNCM I-3446. Environ Microbiol 2016;18:2185–2195.
-
41 Hyde R, Taylor PM, Hundal HS: Amino acid transporters: roles in amino acid sensing and signalling in animal cells. Biochem J 2003; 373:1–18.
-
42 Morán-Ramos S, Ocampo-Medina E, Gutier- rez-Aguilar R, et al: An amino acid signature associated with obesity predicts 2-year risk of hypertriglyceridemia in school-age children. Sci Rep 2017;7:5607.
-
43 Melnik BC: Milk – a nutrient system of mammalian evolution promoting mTORC1-dependent translation. Int J Mol Sci 2015;16: 17048–17087.
-
44 Semba RD, Trehan I, Gonzalez-Freire M, et al: Perspective: the potential role of essential amino acids and the mechanistic target of rapamycin complex 1 (mTORC1) pathway in the pathogenesis of child stunting. Adv Nutr 2016;7:853–865.
-
45 Melnik BC: Excessive leucine-mTORC1-signalling of cow milk-based infant formula: the missing link to understand early childhood obesity. J Obes 2012;2012:197653.
-
46 Storelli G, Defaye A, Erkosar B, et al: Lactobacillus plantarum promotes Drosophila systemic growth by modulating hormonal sig- nals through TOR-dependent nutrient sensing. Cell Metab 2011;14:403–414.
-
47 Murphy K, Curley D, O’Callaghan TF, et al: The composition of human milk and infant faecal microbiota over the first three months of life: a pilot study. Sci Rep 2017;7:40597.