Meeting the Iron Needs of Low and Very Low Birth Weight Infants
Key Messages
- Infants with low birth weight (<2,500 g) are at high risk of iron deficiency and poor health outcomes.
- Delayed umbilical cord clamping and improved iron intake in low birth weight infants improve long-term health outcomes.
Keywords
Iron · Low birth weight · Preterm
Abstract
Low birth weight (LBW), defined as a birth weight of <2,500 g, affects 16% of all newborns and is a risk factor for impaired neurodevelopment as well as adverse cardiovascular and metabolic outcomes, including hypertension. LBW infants include both term, small for gestational age infants and pre-term infants. Most LBW infants have only marginally LBW (2,000–2,500 g). Recent advances in neonatal care have significantly improved the survival of very LBW (VLBW) infants (<1,500 g). LBW infants are at high risk of iron deficiency due to low iron stores at birth and higher iron requirements due to rapid growth. Using a factorial approach, iron requirements of LBW infants have been estimated to be 1–2 mg/kg/ day, which is much higher than the requirements of term, normal birth weight infants, who need almost no dietary iron during the first 6 months of life. In VLBW infants, blood losses and blood transfusions related to neonatal intensive care, as well as erythropoietin treatment, will greatly influence iron status and iron requirements. The timing of umbilical cord clamping at birth is of great importance for the amount of blood transfused from the placenta to the newborn and thereby total body iron. Delayed cord clamping of LBW infants is associated with less need for blood transfusion, less intraventricular hemorrhage, and less necrotizing enterocolitis. Randomized controlled trials have shown that an iron intake of 1–3 mg/kg/day (1–2 mg for marginally LBW and 2–3 mg for VLBW) is needed to effectively prevent iron deficiency. There is some recent evidence that these levels of iron intake will prevent some of the negative health consequences associated with LBW, especially behavioral problems and other neurodevelopmental outcomes and possibly even hypertension. However, it is also important to avoid excessive iron intakes which have been associated with adverse effects in LBW infants.
Background
Low birth weight (LBW), defined as a weight of <2,500 g at birth, regardless of gestational age, is a major risk factor for poor short- and long-term health outcomes. According to the World Health Organization, the global prevalence of LBW is 15.5%, which means that 20 million LBW infants are born each year [1] . The prevalence of LBW is higher in low-income countries and ranges from 4–9% in Europe to 28% in South Asia [2].
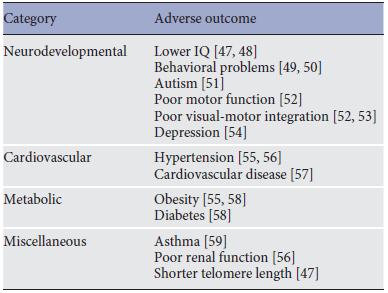
LBW is the leading cause of neonatal mortality worldwide and survivors are at high risk of impaired health later in life, including poor neurodevelopment and adverse cardiovascular/metabolic outcomes. Observed adverse health outcomes in children born with LBW are outlined in Table 1.
LBW infants include both term, small for gestational age infants and preterm infants. Most LBW infants have only marginally LBW (2,000–2,500 g). Recent advances in neonatal care have significantly improved the survival of very LBW (VLBW) infants (<1,500 g).
Recent data suggests that early nutrition may be one of the most important factors that can be modified in clinical practice in order to minimize later morbidity in LBW infants and iron is a critical nutrient in this context.
Iron
Iron is an essential nutrient which is required for heme synthesis, oxygen transport, and many enzyme functions, especially cellular energy metabolism. LBW infants are at high risk of iron deficiency (ID) due to low iron stores at birth, higher iron requirements due to rapid growth, and in the case of VLBW infants, iron losses due to frequent blood samplings during neonatal intensive care. ID should be avoided since it may have adverse effects on brain development. However, in contrast to most other nutrients, there is no mechanism for iron excretion from the human body and iron is a highly reactive pro-oxidant as well as an important substrate for pathogens, so excessive iron supplementation of infants may have adverse effects [3] .
The combination of hemoglobin and ferritin is often used as a measure of iron status in children. Age-specific cutoffs for iron status indicators, including hemoglobin and ferritin, should be used for LBW infants since major physiological changes occur in iron status and red cell morphology during early development (Table 2).
The main public health problem associated with ID in childhood is the risk of poor neurodevelopment. Animal experiments have determined that iron is essential for several aspects of brain development including myelin formation, monoamine synthesis and function, neuronal and glial energy metabolism as well as neuronal growth and arborization [4] . A number of case-control studies in children have shown a consistent association between iron deficiency anemia (IDA) in infancy and long-lasting poor cognitive and behavioral performance up to adolescence [5] . A meta-analysis of 17 randomized controlled clinical trials in children of various ages showed that iron supplementation had a positive effect on mental development indices [6] . However, there is no convincing evidence that iron supplements improve motor or mental development in young children with IDA [7] , suggesting that prevention is a more effective strategy. A meta-analysis based on a very small number of studies showed that preventive iron supplements in infancy (starting at 0–6 months of life) had a positive effect, at least on motor development [8].
Table 1. Adverse health outcomes associated with low birth weight
On the other hand, unnecessary iron supplementation of iron-replete infants may have adverse effects, including increased risk of infections and impaired growth [9] . Iron is an essential nutrient not only for humans but also for almost all living organisms, including pathogenic bacteria and parasites. It has been shown that iron supplementation of young children in malarious regions can increase malaria-related morbidity and mortality if not accompanied by active anti-malaria measures [10, 11] . A possible mechanism for adverse effects of dietary iron may be changes in the gut microbiota. A recent trial in infants showed that iron-fortified porridge resulted in increased abundance of Firmicutes and Escherichia/Shigella, while bifidobacteria decreased [12] .
Since iron cannot be extracted from the body, intestinal iron absorption is strictly regulated. Preterm VLBW infants with a low postnatal iron intake have a capacity for a relatively high fractional iron absorption: 25–40% from iron supplements given between feedings [13, 14] and 11–27% from iron-fortified formula [15, 16] , suggesting that they are able to upregulate intestinal iron absorption when needed. It is less clear whether infants are capable of downregulating iron absorption enough when they are iron sufficient, and one study suggested that this might not be the case in infants up to 6 months of age [17] . Studies are lacking on iron bioavailability from multinutrient-fortified human milk but it may be higher than from preterm formula since, in term infants, iron absorption is significantly higher from human milk than from infant formula [18] .
Table 2. Recommended cutoffs for the diagnosis of iron overload, iron deficiency, and anemia in low birth weight infants at different ages [46]

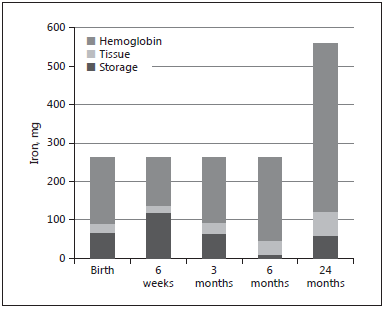
Fig. 1. Body iron compartments and total body iron in a term infant with a birth weight of 3,500 g.
Estimated Iron Requirements
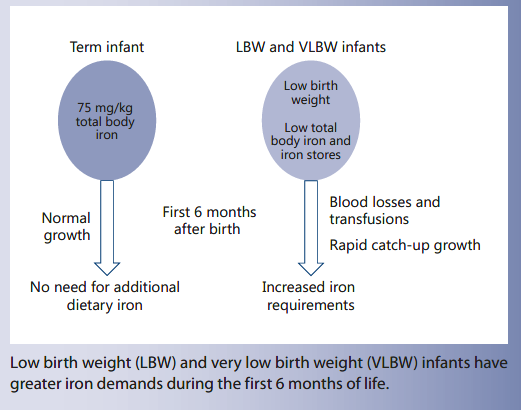
Total body iron at birth is about 75 mg/kg [19] , most of which is found in hemoglobin, and a healthy, term newborn also has some iron stores, corresponding to about 25% of total body iron ( Fig. 1 ). The newborn has a high hemoglobin concentration in blood (average 170 g/L), which is an adaptation to the hypoxic intrauterine environment. Oxygen saturation increases sharply from birth and, consequently, erythropoiesis is downregulated and the hemoglobin level falls to about 120 g/L during the first 6 postnatal weeks [20] . During this period, iron from senescent erythrocytes is transferred from hemoglobin to iron stores, which thereby increase in size. During the following months, as the baby grows and expands its blood volume, iron is gradually transferred back to the hemoglobin compartment until the iron stores are depleted. This means that the normal, term newborn has virtually no need of dietary iron during the first 6 months of life ( Fig. 1 ), which is fortunate since breast milk has a very low concentration of iron (0.3 mg/L) [21, 22].
Two approaches can be used to estimate iron requirements of LBW infants. The first is the factorial approach, i.e., calculating the theoretical iron requirements based on expected growth rate and the iron content of the different body compartments. The second approach is to make recommendations based on interventional trials that have been performed in LBW infants (see Effects of Interventions section).
LBW infants have lower iron stores at birth due to their lower birth weight. As shown in Figure 2 , a LBW infant with a birth weight of 2,000 g has higher iron requirements during the first months of life due to more rapid postnatal growth. The factorial approach, assuming an average body weight of 7.5 kg at 6 months, a blood volume of 80 mL/kg and tissue iron of 7 mg/kg indicate that iron stores of an infant with a birth weight of 2,000 g would be depleted within 6–12 weeks after birth and that the requirement of absorbed iron from 6 weeks to 6 months is 0.12 mg/kg/day. Assuming an average bioavailability of 10%, this corresponds to an enteral iron intake of 1.2 mg/ kg/day.
Using a factorial approach, the iron requirements of a VLBW infant with a birth weight of 1 kg have been estimated to reach a maximum of 0.37 mg/kg/day at around term age [16] . This corresponds to an enteral intake of 1.4–2 mg/kg/day assuming 20–27% absorption. However, such estimations of iron requirements have not considered blood losses, blood transfusions, or erythropoie-tin treatment.
In VLBW infants, blood losses and blood transfusions related to neonatal intensive care, as well as erythropoietin treatment, will greatly influence iron status and iron requirements. Phlebotomy losses commonly amount to 6 mg/kg of iron per week [23] and in some cases much more. Each red blood cell transfusion typically adds 8 mg/ kg of iron and hepatic iron stores as well as serum ferritin concentrations in preterm infants are highly correlated to the number of blood transfusions received [24].
Erythropoietin therapy has been advocated as a way to reduce the need for red blood cell transfusions in VLBW infants and is used in some centers. However, this treatment greatly increases iron requirements and high doses of oral or parenteral iron are therefore recommended as an adjunct to this therapy. Factorial calculations suggest that parenteral iron requirements of VLBW infants would be 0.2–0.37 mg/kg/day [16].
However, parenteral iron has been given in much higher doses, up to 3 mg/kg/day, in erythropoietin trials. Some studies showed that 6 mg/kg/ day of enteral iron supplements is as effective as parenteral iron in this context [25] . There are insufficient data on safety of high doses of iron in combination with erythropoietin, but a Cochrane meta-analysis showed that early erythropoietin treatment, which includes supplemental iron, increases the risk of retinopathy of prematurity [26].
The timing of umbilical cord clamping at birth is of great importance for the amount of blood transfused from the placenta to the newborn and thereby total body iron. We and others have shown that delayed cord clamping increases iron stores and prevents ID at 3–6 months of age in normal birth weight infants [27, 28] . Delayed cord clamping may be even more important in LBW infants. A Cochrane review concluded that delayed cord clamping of preterm infants is associated with less need for blood transfusion, less intraventricular hemorrhage, and less necrotizing enterocolitis [29] .
Effects of Interventions
The risk for LBW infants to develop IDA during the first 6 months of life, without additional dietary iron, is as high as 77% [30] . Intervention studies from the 1970s and 1980s showed that prophylactic iron, in most studies at a dose of 2 mg/kg/day, effectively prevents anemia up to 6 months of age in LBW infants with birth weights ranging from 1,500 to 2,500 g [31] .
There are relatively few randomized intervention trials in LBW infants comparing the effects of different doses of iron supplements or fortification of human milk or infant formula.
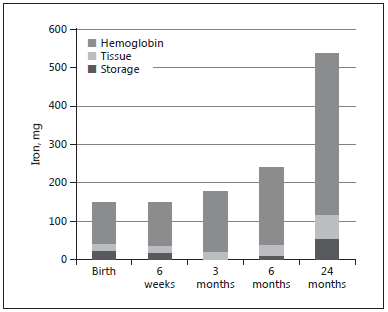
Fig. 2. Body iron compartments and total body iron in a low birth
weight infant with a birth weight of 2,000 g.
In a study by Friel et al. [32] , 58 infants with an average birth weight of 1,500 g were randomized to different infant formulas resulting in iron intakes of 3–6 versus 2– 3 mg/kg/day up to 9 months of age. There was no difference in anemia or neurodevelopment at 12 months between the two groups. However, the group that received the higher amount of iron had higher glutathione peroxidase concentrations (a marker of oxidative stress), lower plasma zinc and copper levels, and a higher incidence of respiratory tract infections, suggesting possible adverse effects of the higher iron intake.
Similarly, Barclay et al. [33] found no effect on hemoglobin of an iron intake of 3.6–6.8 mg/kg/day as compared to 1.0–1.6 mg/kg/day from 2 to 30 weeks postnatal age in infants with an average birth weight of 2,000 g. However, there was a lower erythrocyte superoxide dis-mutase activity in the high iron group, suggesting altered copper metabolism – a possible adverse effect of iron [33].
Taylor and Kennedy [34] randomized 150 VLBW infants to receive 2 mg/kg/day of iron (from fortified breast milk or preterm formula) or an additional 2 mg/kg/day of iron supplements, yielding a total of 4 mg/kg/day, from 2 to 3 weeks of age until 36 weeks postmenstrual age or discharge. No significant effect was observed with regard to the main outcome (hematocrit at 36 weeks) or the number of blood transfusions. Nor was there any significant difference in neonatal morbidity, reticulocyte count, or weight up to 36 weeks. Unfortunately, this study did not include measurements of ferritin or follow-up after discharge.
Griffin et al. [35] randomized 78 mostly VLBW infants (mean birth weight 1,360 g) to receive infant formulas with differing iron contents from hospital discharge until 6 months’ corrected age. The regimens provided iron intakes ranging from 0.8 to 1.2 mg/kg/day. No significant effects were observed between groups with regard to hemoglobin concentrations, ferritin, or the incidence of ID, and hemoglobin concentrations were similar to those of term infants of the same postmenstrual age.
Berglund et al. [36] randomized 285 marginally LBW Swedish infants (2,000–2,500 g) to iron supplements at the following doses: 0 (placebo), 1, or 2 mg/kg/day. The intervention lasted from 6 weeks to 6 months of age. In this population, iron supplements at a dose of 2 mg/kg/ day, compared with placebo, significantly reduced the risk of IDA at 6 months [36] . In the placebo group, 36% developed ID and 10% developed IDA, as compared with 4 and 0%, respectively, in the 2-mg group. Iron supplementation at a rate of 1 or 2 mg/kg/day resulted in differences in iron status, but there was no difference between the two groups in the proportion of infants who developed ID or IDA. Iron supplements did not adversely affect infant growth, infections, or other morbidity in this study. Considering compliance to the intervention as well as iron intakes from other sources (about half of the infants were formula-fed), the investigators found that an actual iron intake of 0.25 mg/kg/day was sufficient to prevent IDA and that an intake of 1 mg/kg/day prevented ID [36] .
Timing of Iron Supplementation of LBW Infants
In order to have a preventive effect on ID, iron supplements should be started before iron stores are depleted, which as a rule of thumb is when the infant has doubled its birth weight, which can be as early as 6 weeks after birth in a moderately LBW infant.
It is generally not recommended to give iron before 2 weeks of age to newborns since there are data suggesting that antioxidant systems are not fully active until that age
[37]. In most studies of iron supplementation of moderately or marginally LBW infants, iron supplementation has been initiated at 4–6 weeks of age.
Two randomized controlled studies in VLBW infants have suggested that an early (2 weeks of age) as compared to late (6–8 weeks) start of iron supplementation results in less need for blood transfusions [38, 39] .
Effects of Early Iron Intake on Neurodevelopment and Health in LBW Infants
There is a severe paucity of studies investigating the effects of early iron intake on neurodevelopment and other long-term health outcomes in LBW infants.
In the small study by Friel et al. [32] (see above), LBW infants were randomized to a normal or high dose of iron and no effect was observed on neurodevelopment at 12 months.
In 2007, Steinmacher et al. [40] published a follow-up study of the VLBW infants in the study by Franz et al. [38] (see above), who were randomized to early versus late iron supplementation. In this follow-up study, children who received early enteral iron supplementation had a significantly lower risk of having abnormal findings on neurological examination at 5 years of age [40] . However, there were no significant differences between groups in test scores for motor or cognitive development. Furthermore, the fact that the infants in the late iron group received more blood transfusions during the neonatal period makes the groups difficult to compare.
A Cochrane review in 2012 investigated the effects of enteral iron supplementation in preterm and LBW infants and found a total of 26 intervention studies. Of the 21 studies comparing iron supplementation with controls, none evaluated neurodevelopmental status as an outcome [41].
Since the Cochrane review, there has been only one new publication on this topic, providing the best current evidence [42] . This was a follow-up of the randomized controlled trial by Berglund et al. [36] , described above, where 385 marginally LBW infants were randomized to receive 0, 1, or 2 mg/kg/day of iron supplements from 6 weeks to 6 months of age. At 3½ years of age, the infants from the original intervention as well as 95 normal birth weight reference children were assessed with a psychometric test (Wechsler Preschool and Primary Scale of Intelligence) and a validated behavioral instrument (the Child Behavior Checklist). There was no difference in cognitive scores between groups, but children in the placebo group had significantly more behavioral problems: 13% compared to 3% in the two iron supplemented groups (p = 0.027) and also compared to 3% in the reference group [42].
An interesting result from the follow-up of this intervention study was that LBW children who received early iron supplementation (1 or 2 mg/kg/day) had lower systolic blood pressure at 7 years (mean difference 2.2 mm Hg, 95% CI 0.3–3.2) as well as a lower risk of having systolic blood pressure within the hypertensive range (OR 0.32; 95% CI 0.11–0.96) [43]. This suggests that the increased risk of hypertension that is observed in children and adults who are born with LBW might at least partly be due to ID and that iron supplementation may reduce this risk. There is a well-known association between ID and pulmonary hypertension in adults and iron has been suggested as a therapy for pulmonary hypertension [44]. A possible mechanism for this blood pressure-lowering effect of iron is an effect on nitric oxide synthesis, which has been observed in a rat model [45].
Conclusions
LBW infants (16% of all newborns globally) are at high risk of ID. Ensuring an adequate iron intake in these infants should be a high priority since it will reduce the risk of ID and IDA and may prevent some of the negative health consequences associated with LBW, especially behavioral problems and other neurodevelopmental outcomes and possibly even hypertension. However, it is also important to avoid excessive iron intakes which have been associated with adverse effects in LBW infants such as an increased risk of infections, impaired zinc and copper status, and increased oxidative stress.
Marginally LBW infants (preterm or term infants with birth weights 2,000–2,500 g) should receive iron supplements of 1–2 mg/kg/day, starting at 2 to 6 weeks of age and continuing to 6 months of age [22] . This dose can safely be recommended for both breastfed and formula-fed infants. In malarious regions, iron supplementation should be combined with anti-malaria measures.
The recommended iron intake of LBW infants with a birth weight of 1,500–2,000 g is 2 mg/kg/day [46] from 2 to 4 weeks of life and this can be achieved initially by using iron-containing human milk fortifier or preterm formula and later (or initially) using iron supplements which should be continued to 6 months of age or possibly longer depending on infant diet.
For VLBW infants, the standard recommendation is a daily iron intake of 2–3 mg/kg/day starting at 2 weeks of age [46] . Infants who receive erythropoietin treatment need a higher dose (up to 6 mg/kg/day) during the treatment period. However, since individual iron status in VLBW infants is highly variable, depending on the number of received blood transfusions and blood losses from phlebotomy, it is recommended to follow these infants with repeated measurements of serum ferritin during the hospital stay. The normal range of ferritin in preterm infants is 35–300 µg/L (Table 2). If ferritin is <35 µg/L, the iron dose should be increased up to 3–4 (or maximum 6) mg/ kg/day may during a limited period. A prolonged dietary iron intake of >3 mg/kg/day should be avoided in most cases because of possible adverse effects. If ferritin is >300 µg/L, which usually is the result of multiple blood transfusions, iron supplementation and fortification should be discontinued until serum ferritin falls below this level. Iron supplements or intake of iron-fortified formula in the recommended doses should be continued also after discharge, at least until 6–12 months of age, depending on diet. Hemoglobin and serum ferritin should be checked at follow-up visits.
Like all infants, LBW infants should receive iron-rich complementary foods from 6 months of age [22] . In addition, delayed umbilical cord clamping, whenever feasible, is recommended for all LBW infants.
These recommendations are based on data from a relatively small number of intervention studies and very few of them investigated the effect on neurodevelopment or other long-term outcomes. More randomized controlled trials with a long-term follow-up are needed to firmly establish the specific iron intakes which will lead to the best health outcomes in this vulnerable group of infants.
Disclosure Statement
The author has no conflicts of interest to report. The writing of this article was supported by Nestlé Nutrition Institute.
References
-
WHO: Care of the preterm and/or low birth weight newborn. 2017. Available from: http:// www.who.int/maternal_child_adolescent/ topics/newborn/care_of_preterm/en/.
-
UNICEF: Low birthweight. 2014. Available from: https://data.unicef.org/topic/nutrition/Low-birthweight/.
-
Domellöf M: Benefits and harms of iron supplementation in iron-deficient and iron-sufficient children. Nestle Nutr Workshop Ser Pediatr Program 2010; 65: 153–162; discussion 162–165.
-
Beard J: Iron deficiency alters brain development and functioning. J Nutr 2003; 133(5 suppl 1):1468S–1472S.
-
Lozoff B, Beard J, Connor J, Barbara F, Georgieff M, Schallert T: Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr Rev 2006; 64:S34–S43; discussion S72–S91.
-
Sachdev H, Gera T, Nestel P: Effect of iron supplementation on mental and motor development in children: systematic review of randomised controlled trials. Public Health Nutr 2005; 8: 117–132.
-
Wang B, Zhan S, Gong T, Lee L: Iron therapy for improving psychomotor development and cognitive function in children under the age of three with iron deficiency anaemia. Cochrane Database Syst Rev 2013:CD001444.
-
Szajewska H, Ruszczynski M, Chmielewska
A:Effects of iron supplementation in non-anemic pregnant women, infants, and young children on the mental performance and psychomotor development of children: a systematic review of randomized controlled trials. Am J Clin Nutr 2010; 91: 1684–1690.
-
Domellöf M: Iron requirements in infancy. Ann Nutr Metab 2011; 59: 59–63.
-
Sazawal S, Black RE, Ramsan M, Chwaya HM, Stoltzfus RJ, Dutta A, et al: Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: community-based, randomised, placebo-controlled trial. Lancet 2006; 367: 133–143.
-
Zlotkin S, Newton S, Aimone AM, Azindow I, Amenga-Etego S, Tchum K, et al: Effect of iron fortification on malaria incidence in infants and young children in Ghana: a randomized trial. JAMA 2013; 310: 938–947.
-
Jaeggi T, Kortman GA, Moretti D, Chassard C, Holding P, Dostal A, et al: Iron fortification adversely affects the gut microbiome, increases pathogen abundance and induces intestinal inflammation in Kenyan infants. Gut 2015; 64: 731–742.
-
Zlotkin SH, Lay DM, Kjarsgaard J, Longley
T:Determination of iron absorption using erythrocyte iron incorporation of two stable isotopes of iron (57Fe and 58Fe) in very low birthweight premature infants. J Pediatr Gastroenterol Nutr 1995; 21: 190–199.
-
Ehrenkranz RA, Gettner PA, Nelli CM, Sherwonit EA, Williams JE, Pearson HA, et al: Iron absorption and incorporation into red blood cells by very low birth weight infants: studies with the stable isotope 58Fe. J Pediatr Gastroenterol Nutr 1992; 15: 270– 278.
-
McDonald MC, Abrams SA, Schanler RJ: Iron absorption and red blood cell incorporation in premature infants fed an iron-fortified infant formula. Pediatr Res 1998; 44: 507–511.
-
Griffin I, Cooke RJ: Iron retention in pre-term infants fed low iron intakes: a metabolic balance study. Early Hum Dev 2010; 86(suppl 1):49–53.
-
Domellöf M, Lonnerdal B, Abrams SA, Her-nell O: Iron absorption in breast-fed infants: effects of age, iron status, iron supplements, and complementary foods. Am J Clin Nutr 2002; 76: 198–204.
-
Fomon SJ, Nelson SE, Ziegler EE: Retention of iron by infants. Annu Rev Nutr 2000; 20: 273–290.
-
Widdowson EM, Southgate DA, Hey E: Fetal growth and body composition; in Lindblad BS (ed): Perinatal Nutrition. Bristol-Myers Nutrition Symposia. 6. New York, Academic Press, 1988, pp 3–14.
-
Domellöf M: Iron requirements, absorption and metabolism in infancy and childhood. Curr Opin Clin Nutr Metab Care 2007; 10: 329–335.
-
Domellöf M, Hemell O, Dewey KG, Cohen RJ, Lonnerdal B: Factors influencing concentrations of iron, zinc, and copper in human milk. Adv Exp Med Biol 2004; 554: 355– 358.
-
Domellöf M, Braegger C, Campoy C, Co-lomb V, Decsi T, Fewtrell M, et al: Iron requirements of infants and toddlers. J Pediatr Gastroenterol Nutr 2014; 58: 119–129.
-
Obladen M, Sachsenweger M, Stahnke M: Blood sampling in very low birth weight infants receiving different levels of intensive care. Eur J Pediatr 1988; 147: 399–404.
-
Ng PC, Lam CW, Lee CH, To KF, Fok TF, Chan IH, et al: Hepatic iron storage in very low birthweight infants after multiple blood transfusions. Arch Dis Child Fetal Neonatal Ed 2001; 84:F101–F105.
-
Ridley FC, Harris J, Gottstein R, Emmerson AJ: Is supplementary iron useful when pre-term infants are treated with erythropoietin? Arch Dis Child 2006; 91: 1036–1038.
-
Ohlsson A, Aher SM: Early erythropoietin for preventing red blood cell transfusion in preterm and/or low birth weight infants. Cochrane Database Syst Rev 2012; 9:CD004863.
-
Andersson O, Hellstrom-Westas L, Anders-son D, Domellöf M: Effect of delayed versus early umbilical cord clamping on neonatal outcomes and iron status at 4 months: a ran-domised controlled trial. BMJ 2011; 343: d7157.
-
McDonald SJ, Middleton P: Effect of timing of umbilical cord clamping of term infants on maternal and neonatal outcomes. Co-chrane Database Syst Rev 2008;CD004074.
-
Rabe H, Diaz-Rossello JL, Duley L, Dow-swell T: Effect of timing of umbilical cord clamping and other strategies to influence placental transfusion at preterm birth on maternal and infant outcomes. Cochrane Database Syst Rev 2012; 8:CD003248.
-
Lundström U, Siimes MA, Dallman PR: At what age does iron supplementation become necessary in low-birth-weight infants? J Pediatr 1977; 91: 878–883.
-
Doyle JJ, Zipursky A: Neonatal blood disorders; in Sinclair JC, Bracken MB (eds): Effective Care of the Newborn Infant. Oxford, Oxford University Press, 1992, pp 425–453.
-
Friel JK, Andrews WL, Aziz K, Kwa PG, Lep-age G, L’Abbe MR: A randomized trial of two levels of iron supplementation and developmental outcome in low birth weight infants. J Pediatr 2001; 139: 254–260.
-
Barclay SM, Aggett PJ, Lloyd DJ, Duffty P: Reduced erythrocyte superoxide dismutase activity in low birth weight infants given iron supplements. Pediatr Res 1991; 29: 297–301.
-
Taylor TA, Kennedy KA: Randomized trial of iron supplementation versus routine iron intake in VLBW infants. Pediatrics 2013; 131: e433–e438.
-
Griffin IJ, Cooke RJ, Reid MM, McCormick KP, Smith JS: Iron nutritional status in pre-term infants fed formulas fortified with iron. Arch Dis Child Fetal Neonatal Ed 1999;81: F45–F49.
-
Berglund S, Westrup B, Domellöf M: Iron supplements reduce the risk of iron deficiency anemia in marginally low birth weight infants. Pediatrics 2010; 126:e874–e883.
-
Berger HM, Mumby S, Gutteridge JM: Ferrous ions detected in iron-overloaded cord blood plasma from preterm and term babies: implications for oxidative stress. Free Radic Res 1995; 22: 555–559.
-
Franz AR, Mihatsch WA, Sander S, Kron M, Pohlandt F: Prospective randomized trial of early versus late enteral iron supplementation in infants with a birth weight of less than 1,301 g. Pediatrics 2000; 106: 700–706.
-
Berseth CL, Van Aerde JE, Gross S, Stolz SI, Harris CL, Hansen JW: Growth, efficacy, and safety of feeding an iron-fortified human milk fortifier. Pediatrics 2004; 114: e699–e706.
-
Steinmacher J, Pohlandt F, Bode H, Sander S, Kron M, Franz AR: Randomized trial of early versus late enteral iron supplementation in infants with a birth weight of less than 1301 grams: neurocognitive development at 5.3 years’ corrected age. Pediatrics 2007; 120: 538–546.
-
Mills RJ, Davies MW: Enteral iron supplementation in preterm and low birth weight infants. Cochrane Database Syst Rev 2012: CD005095.
-
Berglund SK, Westrup B, Hägglöf B, Hernell O, Domellöf M: Effects of iron supplementation of LBW infants on cognition and behavior at 3 years. Pediatrics 2013; 131: 47–55.
-
Lindberg J, Norman M, Westrup B, Domellöf M, Berglund SK: Lower systolic blood pressure at age 7 y in low-birth-weight children who received iron supplements in infancy: results from a randomized controlled trial. Am J Clin Nutr 2017; 106: 475–480.
-
Howard LS, Watson GM, Wharton J, Rhodes CJ, Chan K, Khengar R, et al: Supplementation of iron in pulmonary hypertension: rationale and design of a phase II clinical trial in idiopathic pulmonary arterial hypertension. Pulm Circ 2013; 3: 100–107.
-
Wu G, Meininger CJ: Regulation of nitric oxide synthesis by dietary factors. Annu Rev Nutr 2002; 22: 61–86.
-
Domellöf M: Nutritional care of premature infants: microminerals. World Rev Nutr Diet 2014; 110: 121–139.
-
Strohmaier J, van Dongen J, Willemsen G, Nyholt DR, Zhu G, Codd V, et al: low birth weight in MZ twins discordant for birth weight is associated with shorter telomere length and lower IQ, but not anxiety/depression in later life. Twin Res Hum Genet 2015; 18: 198–209.
-
Kormos CE, Wilkinson AJ, Davey CJ, Cunningham AJ: Low birth weight and intelligence in adolescence and early adulthood: a meta-analysis. J Public Health (Oxf) 2014; 36: 213–224.
-
Gray RF, Indurkhya A, McCormick MC: Prevalence, stability, and predictors of clinically significant behavior problems in low birth weight children at 3, 5, and 8 years of age. Pediatrics 2004; 114: 736–743.
-
Fan RG, Portuguez MW, Nunes ML: Cognition, behavior and social competence of pre-term low birth weight children at school age. Clinics (Sao Paulo) 2013; 68: 915–921.
-
Wang C, Geng H, Liu W, Zhang G: Prenatal, perinatal, and postnatal factors associated with autism: a meta-analysis. Medicine (Baltimore) 2017; 96:e6696.
-
Geldof CJ, van Hus JW, Jeukens-Visser M, Nollet F, Kok JH, Oosterlaan J, et al: Deficits in vision and visual attention associated with motor performance of very preterm/very low birth weight children. Res Dev Disabil 2016; 53–54: 258–266.
-
Perez-Roche T, Altemir I, Gimenez G, Prieto E, Gonzalez I, Pena-Segura JL, et al: Effect of prematurity and low birth weight in visual abilities and school performance. Res Dev Disabil 2016; 59: 451–457.
-
Loret de Mola C, de Franca GV, Quevedo Lde A, Horta BL: Low birth weight, preterm birth and small for gestational age association with adult depression: systematic review and meta-analysis. Br J Psychiatry 2014; 205: 340– 347.
-
Zarrati M, Shidfar F, Razmpoosh E, Nezhad FN, Keivani H, Hemami MR, et al: Does low birth weight predict hypertension and obesity in schoolchildren? Ann Nutr Metab 2013; 63: 69–76.
-
Luyckx VA, Perico N, Somaschini M, Man-fellotto D, Valensise H, Cetin I, et al: A developmental approach to the prevention of hypertension and kidney disease: a report from the Low Birth Weight and Nephron Number Working Group. Lancet 2017;390:424–428.
-
Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ: Weight in infancy and death from ischaemic heart disease. Lancet 1989; 2: 577–580.
-
Jornayvaz FR, Vollenweider P, Bochud M, Mooser V, Waeber G, Marques-Vidal P: Low birth weight leads to obesity, diabetes and increased leptin levels in adults: the CoLaus study. Cardiovasc Diabetol 2016; 15: 73.
-
Matheson MC, AL DO, Burgess JA, Giles GG, Hopper JL, Johns DP, et al: Preterm birth and low birth weight continue to increase the risk of asthma from age 7 to 43. J Asthma 2016; 1–8.