Lactose Intolerance: Common Misunderstandings
Key Messages
- Lactose intolerance is often confused with cow’s milk allergy by patients and parents.
- A better knowledge of the differences between these clinical conditions could limit misunderstandings in the diagnostic approach and management.
Keywords
Adverse food reactions · Food intolerance · Lactase · Cow’s milk allergy · Breath test
Abstract
Lactose intolerance primarily refers to a syndrome having different symptoms upon the consumption of foods containing lactose. It is one of the most common form of food intolerance and occurs when lactase activity is reduced in the brush border of the small bowel mucosa. Individuals may be lactose intolerant to varying degrees, depending on the severity of these symptoms. When lactose is not digested, it can be fermented by gut microbiota leading to symptoms of lactose intolerance that include abdominal pain, bloating, flatulence, and diarrhea with a considerable intraindividual and interindividual variability in the severity of clinical manifestations. These gastrointestinal symptoms could be similar to cow’s milk allergy and could be wrongly labeled as symptoms of “milk allergy.” There are important differences between lactose intolerance and cow’s milk allergy; therefore, a better knowledge of these differences could limit misunderstandings in the diagnostic approach and in the management of these conditions.
Introduction
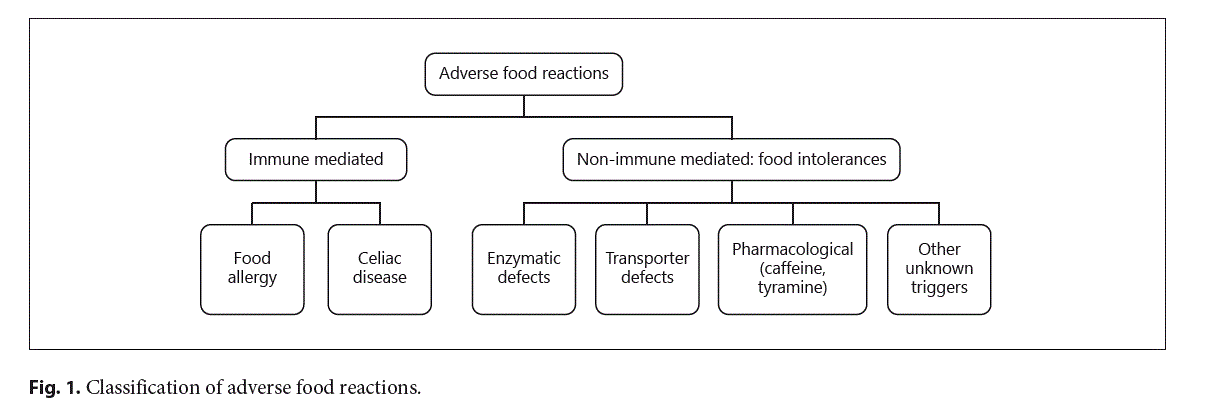
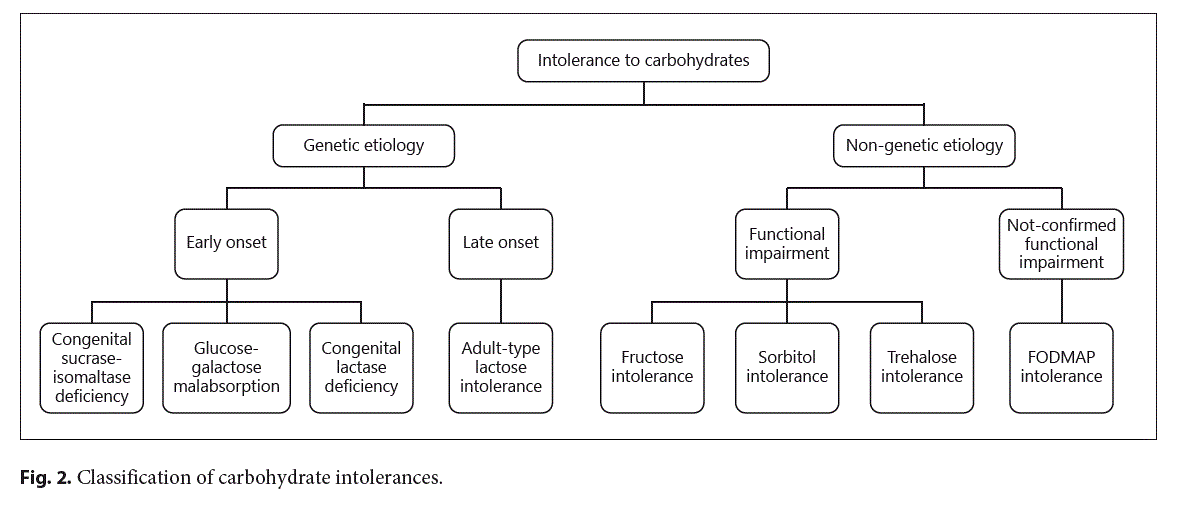
The signs and symptoms of adverse food reactions (AFRs) in children derive from several mechanisms (see Fig. 1) [1]. These mechanisms can be triggered by different components of the same food. Immune-mediated re- actions (i.e., food allergy, celiac disease) are elicited by food proteins, whereas the vast majority of non-immune-mediated AFRs derive from carbohydrate intolerances (see Fig. 2). The most common carbohydrate intolerance in the pediatric age is lactose intolerance. During infancy, lactose accounts for most of the dietary carbohydrates. Lactose is a disaccharide, which is present in many dairy products, composed by galactose linked to glucose via a β-1→4 glucosidic bond. Lactose is hydrolyzed by β-galactosidase (lactase) bound to the small intestine brush border membrane, then the monosaccharides glucose and galactose are both actively absorbed in the small intestine. Lactose intolerance primarily refers to a syndrome having different intestinal or extraintestinal symptoms upon the consumption of foods containing lactose that derives from an insufficient level of lactase activity in the brush border of the small bowel mucosa [2].
Three Types of Lactose Intolerance
Different factors cause the lactase deficiency underlying each type:
- Congenital lactase deficiency (CLD): an extremely rare autosomal recessive disease characterized by absent or reduced enzymatic activity from birth.
- Primary lactose intolerance or adult-type lactase deficiency: a common autosomal recessive condition resulting from a developmentally regulated change of the lactase gene expression.
- Secondary lactase deficiency: a transient condition deriving from intestinal damage secondary to several dis- eases such as infections, food allergy, celiac disease, small bowel bacterial overgrowth, Crohn’s disease, or radiation/chemotherapy-induced enteritis.
CLD is a rare (only a few cases have been described) and severe intestinal autosomal recessive disease, within the group of congenital diarrheal disorders, caused by theabsenceoflactaseactivityfrombirth(OMIM 223000) [3, 4]. This condition must be distinguished from the developmental lactose intolerance that could be ob- served in premature infants. These subjects may have reduced levels of lactase because small intestinal lactase-expressing enterocytes develop later in the third trimester. The main symptoms of CLD are watery diarrhea, intestinal meteorism, and malnutrition, beginning on the first days after birth with the onset of lactation with breast milk or lactose-containing formula. Symptoms disappear when patients change to a lactose-free diet. The typical feature of CLD is the absence or very low levels of lactase expression deriving from a mutation in lactase phlorizin hydrolase gene (LPH) located on 2q21.3 [5, 6]. Most CLD cases have been described in Finland, where the disorder is enriched due to a founder effect and genetic drift [5, 7]. Premature stop codons and a truncated protein as a result of frame shifts, missense mutations in the coding region of LPH, or exon duplication are the most common genotypes identified in these patients [7–10]. Some other cases include mutations leading to single amino acid substitutions that can interfere with the proper maturation and function of LPH [7, 11]. More recently, severe forms of CLD elicited by mutations in the LPH gene that occur in either a compound heterozygous or homozygous pattern of inheritance have been described [3].
In primary lactose intolerance, intestinal lactase expression falls off sharply, making dairy products difficult to digest later in childhood or adolescence. It is the most common type of lactose intolerance and it is genetically determined. Approximately 70% of the global adult population are lactase non-persistent (hypolactasia). The global distribution and the age at which lactase expression declines vary with ethnicity. In South America, Africa, and Asia, more than 50% are lactase non-persistent. The condition is also common in Mediterranean or Southern European populations. In some Asian countries, up to 100% are lactase non-persistent. In the United States, the percentage of lactase non-persistence varies with ethnic origin with the lowest percentage in the population of European origin and the highest percentage in Hispanics and in the Afro-American population. People who develop primary lactose intolerance start life producing plenty of lactase, a necessity for infants, who get all their nutrition from milk. As children replace milk with other foods, their lactase production decreases. Children of African, Asian, or Hispanic descent may experience symptoms beginning between the age of 2 and 3 years, whereas subjects of European and American descent typically do not develop symptoms of lactose intolerance until later in childhood (5–6 years of age) or adolescence [12–14]. Lactase persistence is inherited as a dominant Mendelian trait [15]. The genetic trait of persistence of intestinal lactase expression can be caused by five or more independent single nucleotide variants in a regulatory region (a transcriptional enhancer) upstream of the lactase gene. One of these, −13910*T (rs4988235) is responsible for most cases of lactase persistence in Caucasian individuals, others such as −13907*G (rs41525747), −13915*G (rs41380347), −14009*G (rs869051967), and −14010*C (rs145946881) are found at variable frequencies in the Middle East and Africa [16, 17]. Several individual variables can influence the development of symptoms in non-persistence lactase subjects: dose of lactose in diet, intestinal transit time, lactase expression, distribution and fermentation ability of gut microbiota, sensitivity towards chemical and mechanical stimulation of the gut, and psychological factors [18–20]. Adaptation of gut microbiota, assuming a growing dose of lactose, with increase of bacterial β-galactosidase activity is recognized as a cause of symptom reduction in lactose intolerance [21, 22].
Lastly, virtually all pathological conditions that cause small intestine damage can induce a reduction in lactase expression determining a secondary and transient lactase deficiency. Among the diseases associated with secondary lactose intolerance there are celiac disease, small bacterial overgrowth, and Crohn’s disease. Treatment of the underlying disorder may restore lactase levels and improve signs and symptoms, though it can take time. Abdomen radiation therapy or chemotherapy could also lead to lactose intolerance. Cow’s milk allergy (CMA) can cause severe enteropathy with secondary lactase deficiency. In these patients, there may be an overlap of gastrointestinal symptoms due to CMA and lactose intolerance. Therefore, the same food, such as cow’s milk, can lead to an adverse reaction through different mechanisms.
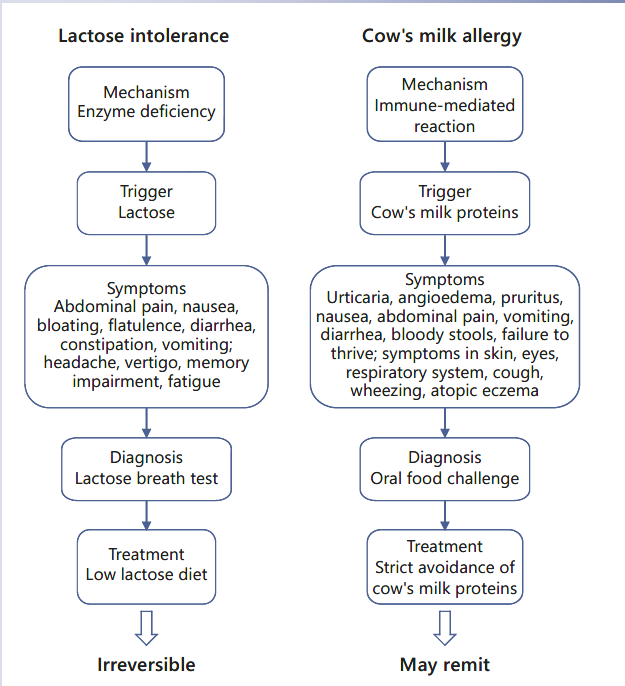
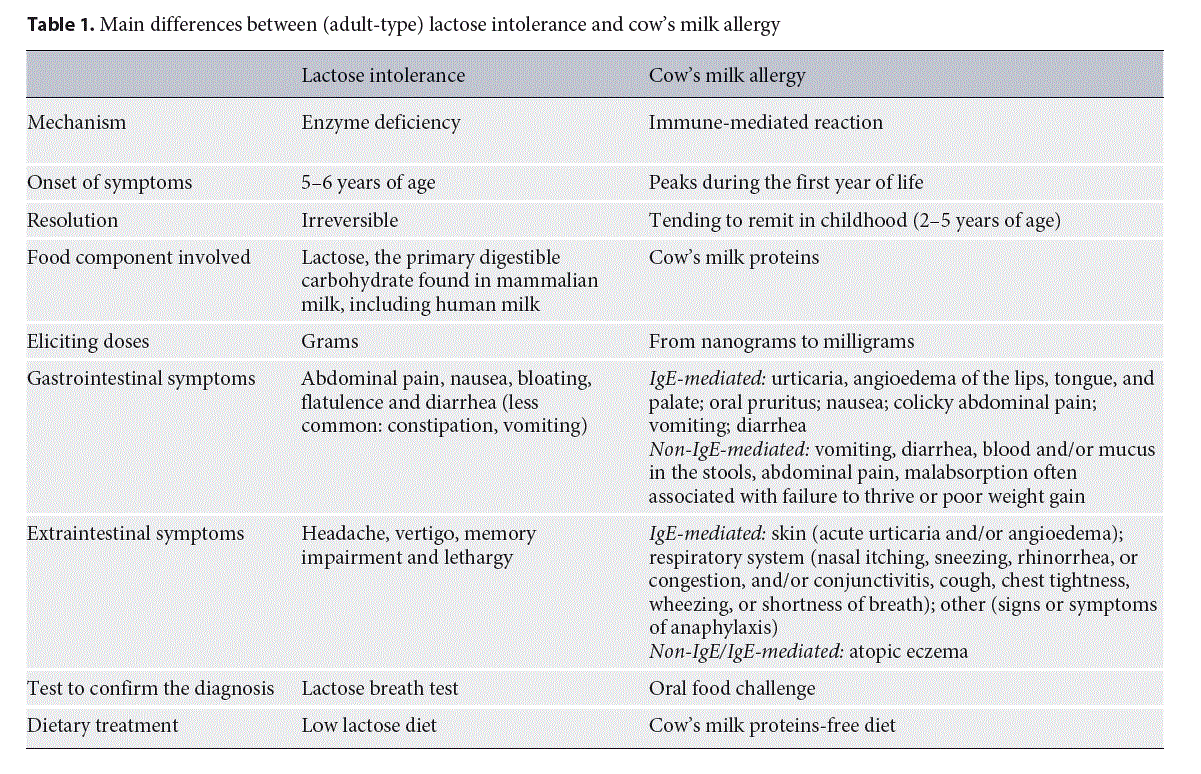
Differences between Lactose Intolerance and CMA
Frequently, among both patients and physicians, there is confusion between lactose intolerance and CMA, which could result in unnecessary dietary restriction or avoidable reactions. “Milk allergy,” “milk intolerance,” and “lactose intolerance” are often used by patients and their parents without a clear sense of the different meanings, understanding of the different mechanisms that underlie them, or the dietary implications of the diagnosis. The management of these conditions is distinctly different, and inappropriate recognition or management may have significant implications for the patient [23].
Lactose intolerance results from a reduced ability to digest lactose, a sugar. As explained above, lactose intolerance is a “non-immune-mediated AFR,” while CMA is one of the most common forms of food allergy (“immune-me- diated AFR”) in particular in the first years of life. CMA may be due to immunoglobulin E (IgE), non-IgE mediated, or mixed reactions. After food intake, IgE-mediated re- actions typically occur within 2 h, whereas non-IgE-mediated reactions develop after 2–48 h or some days after the food ingestion [24]. In particular, the symptoms of non- IgE-mediated CMA are frequently wrongly labeled as symptoms of intolerance. The main differences between CMA and lactose intolerance are summarized in Table 1.
Clinical Symptoms of Lactose Intolerance
Non-digested lactose in the intestinal tract drives fluids into the gut lumen through an osmotic force, causing osmotic diarrhea. Moreover, gut microbiota fermented lactose, producing volatile fatty acids and gases (hydrogen, methane, and carbon dioxide). All these events are responsible for the clinical symptoms, such as distension of the small bowel, non-focal abdominal pain associated with bloating and flatulence, nausea, increased gut motility, and diarrhea [25]. These symptoms usually develop from 30 min to 2 h after the ingestion of lactose-containing foods. Food intolerances have long been reported by patients with functional gastrointestinal disorders; however, randomized controlled trials are lacking in this area [26]. Extraintestinal symptoms, such as headache, vertigo, memory impairment, and lethargy, have been described in up to 20% of subjects with carbohydrate intolerance [27]. These systemic symptoms could be the result of toxic metabolites, produced by sugar fermentation of colonic bacteria that can alter cell-signaling mechanisms [28]. However, it is unclear whether these atypical symptoms are directly due to lactose ingestion or related to the presence of the so-called “functional disease,” frequently accompanied by multiple somatic complaints [17].
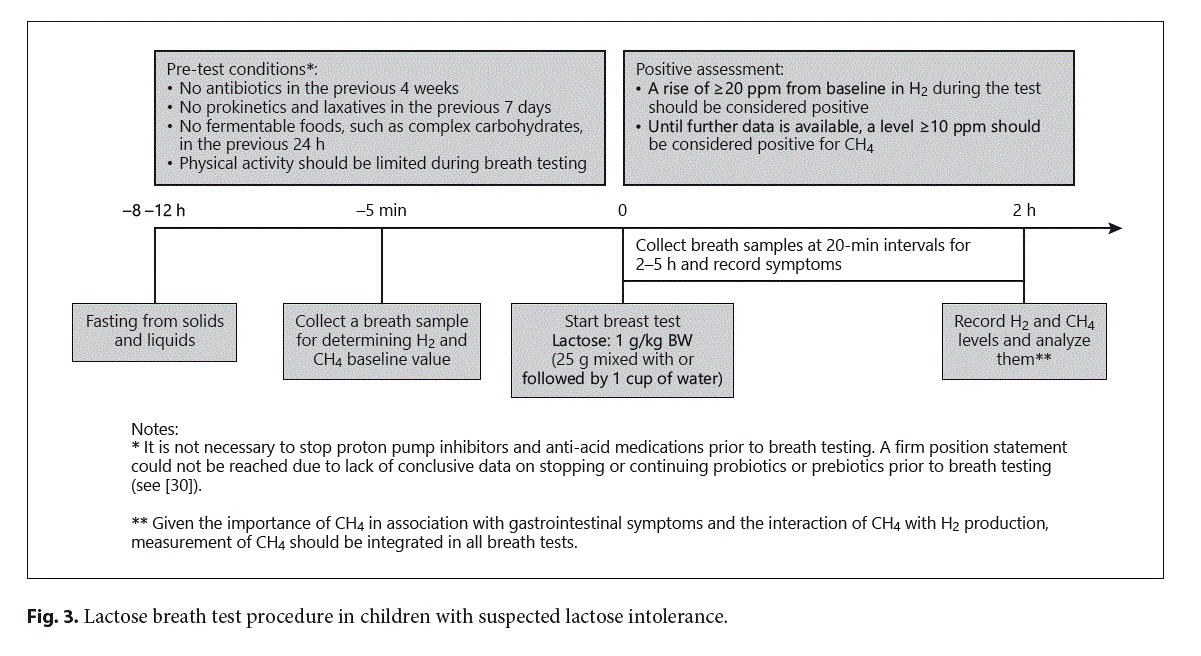
Diagnostic Approach to a Pediatric Patient with Suspected Lactose Intolerance
Genetic testing for mutations of the LPH gene should be performed whenever CLD is suspected in infants with typical symptoms and a positive response to dietary elimination of lactose [29]. In secondary lactase deficiency, a good clinical history often reveals the relationship be- tween lactose ingestion and symptoms. The mainstays of adult-type lactose intolerance diagnosis are anamnesis and the lactose breath test (LBT) [17]. The LBT is a rapid, non-invasive test that allows measuring the content of hydrogen in the expired air. The dose of lactose administered is 1 g/kg in children. Although high doses of lactose (≥50 g) have been used for LBT, 25 g (equivalent of 500 mL of milk) is within the normal range of consumption and is the recommended dose after 8–12 h of fasting [30]. All breath testing should incorporate measurement of CO2 (or O2) to adjust the breath sample for non-alveolar dilution of exhaled air [31]. Concomitant measurement of CH4 is also required because the detection rate of an early rise in H2 production significantly decreases in excess methane producers [30]. A cutoff increase of H2 of 20 parts per million (ppm) above the baseline level is considered as positive (CH4 ≥10 ppm) (see Fig. 3).
Factors that may produce false-negative or false-positive results include conditions affecting the gut microbiota (e.g., recent use of antimicrobial agents), lack of hydrogen-producing bacteria (10–15% of the population), ingestion of high-fiber diets before the test, small intestinal bacterial overgrowth, or intestinal motility disorders [17, 32].
Another diagnostic test, quite popular in the past, is the lactose tolerance test. In this test, the patient suspected to have lactose intolerance assumes 50 g of lactose dissolved in water. Samples of capillary blood are taken to test the plasma glucose concentration at −5, 0, 15, 30, 45, and 60 min. A maximal plasma-glucose increase of 1.4 mmol/L or higher indicates lactose tolerance [33]. The lactose tolerance test is not sensitive enough; it is also of- ten falsely positive because of lack of an increase of blood glucose concentration attributable to a normal insulin response to the carbohydrate load. Given the high rate of false-negative and false-positive results, this test should not be used and has been replaced by the LBT [34].
The genetic test, identifying single nucleotide polymorphism associated with lactase persistence/non-persistence, is also available. It should be noted that the presence of the lactase non-persistent gene does not imply the simultaneous presence of lactose intolerance that may appear later in life.

Management of Lactose Intolerance and Nutritional Issues
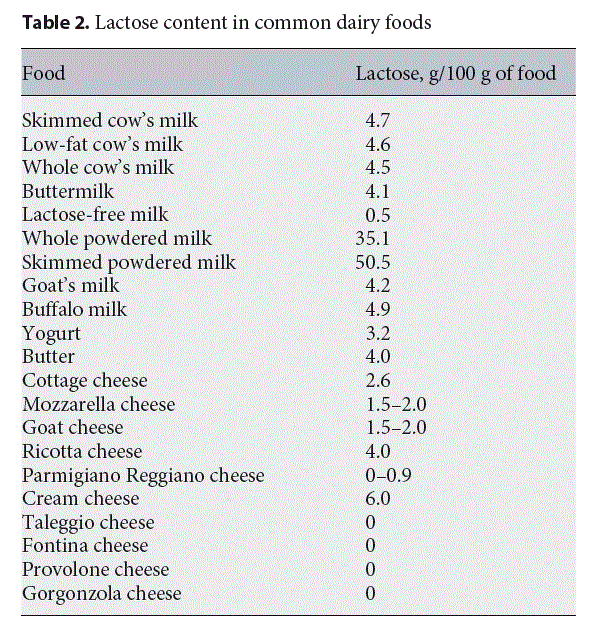
The mainstay of treatment for AFRs is to eliminate the causative food from the diet. In the AFRs induced by CMA, also small protein doses can cause symptoms, so the management is based on the strict avoidance of the cow’s milk-derived allergenic peptides in the diet. On the contrary, a reduction of lactose intake rather than full exclusion is recommended in lactose intolerance, because available data suggest that adolescents and adults can usually ingest up to 12 g of lactose in a single dose (equivalent to 1 cup of milk, corresponding to 240 mL) with no or minimal symptoms [35]. So, in these patients dietary treatment consists only in a low-lactose diet (Tables 2, 3) [2, 35]. There is no scientific evidence to identify the tolerable dose of lactose for children with lactose intolerance. Determining the amounts of lactose that can be tolerated is necessary to develop evidence-based dietary recommendations that meet the needs of the individual. In primary lactose intolerance, lactose-containing dairy products are generally avoided for 2–4 weeks, the time required to induce symptom remission. Then, a gradual reintroduction of dairy products low in lactose up to a threshold dose of individual tolerance should be recommended.
In secondary hypolactasia, a restricted diet is necessary only for a limited time [35]. Concern about lactose intolerance and osmotic diarrhea in the treatment of under- nourished children has led to a restricted use of lactose in these patients. Even in well-nourished children, low-lactose formulas are frequently used in children with persistent diarrhea. It is useful to find a balance where the amount of lactose in food does not induce osmotic diarrhea, but can help to achieve the beneficial effects of lactose. Clinical trials are needed to better define the safe and appropriate lactose dietary levels for moderately and severely undernourished children [36]. In the rare form of CLD, a complete lactose-free diet is required for life.
Enzyme replacement is another therapeutic approach in patients with lactose intolerance that wish to enjoy dairy products. Preliminary data showed an improvement of gastrointestinal symptoms and a decrease of H2 levels at breath test with the administration of 1,500U/day ofβ-galactosidase. However, more data regarding the efficacy of this microbial exogenous enzyme are needed [37]. Other evidence suggested that efficacy of exogenous lactase was obtained from Kluyveromyces lactis, Aspergillus oryzae, or Kluyveromyces lactis [38, 39]. Another strategy involves probiotics that could shape gut microbiota composition. Four-week consumption of a mix probiotic combination (Lactobacillus casei Shirota and Bifidobacterium breve) improved symptoms and decreased H2 production in lactose-intolerant patients. These effects appeared to be persistent for at least 3 months after suspension of probiotic consumption [40], and strain-specific because in a similar study a milk containing L. acidophilus resulted ineffective [41]. A randomized, double-blind, placebo-controlled trial conducted in adult lactose-intolerant patients demonstrated that a 36-day treatment with a highly purified (>95%) short-chain galactooligosaccharide (GOS), designated “RP-G28” (escalating doses from 1.5 to 15 g/day) plus subsequent dairy consumption significantly improved clinical outcomes for lactose digestion and tolerance. These clinical outcomes correlated with a significant modification in gut microbiota composition consisting of an increase in lactose-fermenting Bifidobacterium, Faecalibacterium, Lactobacillus, and Roseburia [42]. Further studies are required to provide high-quality evidence to support or compare the efficacy of all these strategies.
In the management of lactose-intolerant patients, it is important to consider that lactose intolerance can be part of a wider intolerance to variably absorbed, fermentable oligo-di-, monosaccharides and polyols (FODMAPs). This is present in a high percentage of patients with irritable bowel syndrome and this group requires not only restriction of lactose intake but also a low-FODMAP diet to improve gastrointestinal symptoms [17].
“Free” diets are in fashion. In supermarkets tons of products labeled lactose-free can be easily found; there are more and more cafes, ice-cream shops, bakeries, and restaurants offering special menus, where lactose has been banished. Milk consumption is decreasing in the USA and is the lowest in countries with a high prevalence of lactase non-persistence [14]. Indeed, the National Health and Nutrition Examination Survey (NHANES) reported approximately 5% of infants received lactose-reduced formulas in the USA alone be- tween 2003 and 2010, and this trend is increasing [43]. A common rationale for the use of lactose-free infant formulas is that infants are presumed to be lactose intolerant; although there is little or no evidence that lactose-reduced formulas are beneficial [44]. Preliminary evidence shows that elimination of lactose from the infants’ diet is disadvantageous for the development of healthy gut microbiome [45] and a different plasma metabolic profile in lactose-free formula-fed children [46]. A lactose-free diet should be prescribed only when a true diagnosis of lactose tolerance is achieved. A full dairy exclusion diet may also affect other health out- comes. It is important to underline that if dairy products are eliminated, other dietary sources of calcium or calcium supplements need to be provided. The current recommendations for calcium intake are 700 mg/day for children aged 4–9 years, and 1,300 mg/day over 10 years, according to the EFSA guidelines [47]. Educational and commercial efforts to improve calcium and vitamin D intake are now focusing on stimulating the consumption of tolerable amounts of milk, use of lowered lactose-containing foods including hard cheeses, yogurt, and lactose-hydrolyzed milk products.
References
- 1 Lomer MC. Review article: the aetiology, diagnosis, mechanisms and clinical evidence for food intolerance. Aliment Pharmacol Ther. 2015 Feb;41(3):262–75.
- 2 Berni Canani R, Pezzella V, Amoroso A, Coz- zolino T, Di Scala C, Passariello A. Diagnosing and treating intolerance to carbohydrates in children. Nutrients. 2016 Mar;8(3):157.
- 3 Diekmann L, Pfeiffer K, Naim HY. Congenital lactose intolerance is triggered by severe mutations on both alleles of the lactase gene. BMC Gastroenterol. 2015 Mar;15(1):36.
- 4 Berni Canani R, Castaldo G, Bacchetta R, Martín MG, Goulet O. Congenital diarrhoeal disorders: advances in this evolving web of inherited enteropathies. Nat Rev Gastroenterol Hepatol.2015 May;12(5):293–302.
- 5 Järvelä I, Enattah NS, Kokkonen J, Varilo T, Savilahti E, Peltonen L. Assignment of the locus for congenital lactase deficiency to 2q21, in the vicinity of but separate from the lactase-phlorizin hydrolase gene. Am J Hum Genet. 1998 Oct;63(4):1078–85.
- 6 Järvelä I, Torniainen S, Kolho KL. Molecular genetics of human lactase deficiencies. Ann Med. 2009;41(8):568–75.
- 7 Torniainen S, Freddara R, Routi T, Gijsbers C, Catassi C, Höglund P, et al. Four novel mutations in the lactase gene (LCT) underlying congenital lactase deficiency (CLD). BMC Gastroenterol.2009 Jan;9(1):8.
- 8 Kuokkanen M, Kokkonen J, Enattah NS, Ylisaukko-Oja T, Komu H, Varilo T, et al. Mutations in the translated region of the lactase gene (LCT) underlie congenital lactase deficiency. Am J Hum Genet. 2006 Feb;78(2): 339–44.
- 9 Sala Coromina J, Vinaixa Vergés A, Garcia Puig R. [Congenital lactase deficiency: identification of a new mutation]. An Pediatr (Barc). 2015 May;82(5):365–6.
- 10 Uchida N, Sakamoto O, Irie M, Abukawa D, Takeyama J, Kure S, et al. Two novel mutations in the lactase gene in a Japanese infant with congenital lactase deficiency. Tohoku J Exp Med. 2012 May;227(1):69–72.
- 11 Behrendt M, Keiser M, Hoch M, Naim HY. Impaired trafficking and subcellular localization of a mutant lactase associated with congenital lactase deficiency. Gastroenterology. 2009 Jun;136(7):2295–303.
- 12 Born P, Sekatcheva M, Rösch T, Classen M. Carbohydrate malabsorption in clinical routine: a prospective observational study. Hepatogastroenterology. 2006 Sep-Oct; 53(71): 673–7.
- 13 Lomer MC, Parkes GC, Sanderson JD. Review article: lactose intolerance in clinical practice—myths and realities. Aliment Pharmacol Ther. 2008 Jan;27(2):93–103.
- 14 Bayless TM, Brown E, Paige DM. Lactase Non-persistence and lactose intolerance. Curr Gastroenterol Rep. 2017 May;19(5):23.
- 15 Enattah NS, Sahi T, Savilahti E, Terwilliger JD, Peltonen L, Järvelä I. Identification of a variant associated with adult-type hypolactasia. Nat Genet. 2002 Feb;30(2):233–7.
- 16 Liebert A, López S, Jones BL, Montalva N, Gerbault P, Lau W, et al. World-wide distributions of lactase persistence alleles and the complex effects of recombination and selection. Hum Genet. 2017 Nov; 136(11-12): 1445–53.
- 17 Deng Y, Misselwitz B, Dai N, Fox M. Lactose Intolerance in Adults: Biological Mechanism and Dietary Management. Nutrients. 2015 Sep;7(9):8020–35.
- 18 Zhao J, Fox M, Cong Y, Chu H, Shang Y, Fried M, et al. Lactose intolerance in patients with chronic functional diarrhoea: the role of small intestinal bacterial overgrowth. Aliment Pharmacol Ther. 2010 Apr;31(8):892–900.
- 19 Tomba C, Baldassarri A, Coletta M, Cesana BM, Basilisco G. Is the subjective perception of lactose intolerance influenced by the psychological profile? Aliment Pharmacol Ther. 2012 Oct;36(7):660–9.
- 20 Suchy FJ, Brannon PM, Carpenter TO, Fernan- dez JR, Gilsanz V, Gould JB, et al. NIH consensus development conference statement: lactose intolerance and health. NIH Consens State Sci Statements. 2010 Feb;27(2):1–27.
- 21 Johnson AO, Semenya JG, Buchowski MS, Enwonwu CO, Scrimshaw NS. Adaptation of lactose maldigesters to continued milk in- takes. Am J Clin Nutr. 1993 Dec;58(6):879–81.
- 22 Briet F, Pochart P, Marteau P, Flourie B, Arrigoni E, Rambaud JC. Improved clinical tolerance to chronic lactose ingestion in subjects with lactose intolerance: a placebo effect? Gut. 1997 Nov;41(5):632–5.
- 23 Walsh J, Meyer R, Shah N, Quekett J, Fox AT. Differentiating milk allergy (IgE and non-IgE mediated) from lactose intolerance: under- standing the underlying mechanisms and presentations. Br J Gen Pract. 2016 Aug; 66(649):e609–11.
- 24 Turnbull JL, Adams HN, Gorard DA. Review article: the diagnosis and management of food allergy and food intolerances. Aliment Pharmacol Ther. 2015 Jan;41(1):3–25.
- 25 Vandenplas Y. Lactose intolerance. Asia Pac J Clin Nutr. 2015;24 Suppl 1:S9–13.
- 26 Wilson K, Hill RJ. The role of food intolerance in functional gastrointestinal disorders in children. Aust Fam Physician. 2014 Oct;43(10): 686–9.
- 27 Matthews SB, Waud JP, Roberts AG, Camp- bell AK. Systemic lactose intolerance: a new perspective on an old problem. Postgrad Med J. 2005 Mar;81(953):167–73.
- 28 Campbell AK, Matthews SB, Vassel N, Cox CD, Naseem R, Chaichi J, et al. Bacterial metabolic ‘toxins’: a new mechanism for lactose and food intolerance, and irritable bowel syndrome.Toxicology.2010 Dec;278(3):268–76.
- 29 Fazeli W, Kaczmarek S, Kirschstein M, Santer R. A novel mutation within the lactase gene (LCT): the first report of congenital lactase deficiency diagnosed in Central Europe. BMC Gastroenterol. 2015 Jul;15(1):90.
- 30 Rezaie A, Buresi M, Lembo A, Lin H, McCal- lum R, Rao S, et al. Hydrogen and Methane- Based Breath Testing in Gastrointestinal Dis- orders: the North American Consensus. Am J Gastroenterol.2017 May;112(5):775–84.
- 31Goldoni M, Corradi M, Mozzoni P, Folesani G, Alinovi R, Pinelli S, et al. Concentration of exhaled breath condensate biomarkers after fractionated collection based on exhaled CO2 signal. J Breath Res. 2013 Mar;7(1): 017101.
- 32 Däbritz J, Mühlbauer M, Domagk D, Voos N, Henneböhl G, Siemer ML, et al. Significance of hydrogen breath tests in children with suspected carbohydrate malabsorption. BMC Pediatr. 2014 Feb;14(1):59.
- 33 Di Rienzo T, D’Angelo G, D’Aversa F, Campanale MC, Cesario V, Montalto M, et al. Lactose intolerance: from diagnosis to correct management. Eur Rev Med Pharmacol Sci. 2013;17 Suppl 2:18–25.
- 34 Heyman MB; Committee on Nutrition. Lactose intolerance in infants, children, and ado- lescents. Pediatrics. 2006 Sep;118(3):1279–86.
- 35 Usai-Satta P, Scarpa M, Oppia F, Cabras F. Lactose malabsorption and intolerance: what should be the best clinical management? World J Gastrointest Pharmacol Ther. 2012 Jun;3(3):29–33.
- 36 Grenov B, Briend A, Sangild PT, Thymann T, Rytter MH, Hother AL, et al. Undernourished Children and Milk Lactose. Food Nutr Bull. 2016 Mar;37(1):85–99.
- 37 Ibba I, Gilli A, Boi MF, Usai P. Effects of exogenous lactase administration on hydrogen breath excretion and intestinal symptoms in patients presenting lactose malabsorption and intolerance. BioMed Res Int. 2014;2014: 680196.
- 38 Montalto M, Nucera G, Santoro L, Curigliano V, Vastola M, Covino M, et al. Effect of exogenous beta-galactosidase in patients with lactose malabsorption and intolerance: a crossover double-blind placebo-controlled study. Eur J Clin Nutr. 2005 Apr;59(4):489–93.
- 39 Ojetti V, Gigante G, Gabrielli M, Ainora ME, Mannocci A, Lauritano EC, et al. The effect of oral supplementation with Lactobacillus re- uteri or tilactase in lactose intolerant patients: randomized trial. Eur Rev Med Pharmacol Sci. 2010 Mar;14(3):163–70.
- 40 Almeida CC, Lorena SL, Pavan CR, Akasaka HM, Mesquita MA. Beneficial effects of long-term consumption of a probiotic combination of Lactobacillus casei Shirota and Bifidobacterium breve Yakult may persist after suspension of therapy in lactose-intolerant patients. Nutr Clin Pract. 2012 Apr;27(2):247–51.
- 41 Shaukat A, Levitt MD, Taylor BC, MacDonald R, Shamliyan TA, Kane RL, et al. Systematic review: effective management strategies for lactose intolerance. Ann Intern Med. 2010 Jun;152(12):797–803.
- 42 Azcarate-Peril MA, Ritter AJ, Savaiano D, Monteagudo-Mera A, Anderson C, Magness ST, et al. Impact of short-chain galactooligosaccharides on the gut microbiome of lactose- intolerant individuals. Proc Natl Acad Sci USA. 2017 Jan;114(3):E367–75.
- 43 Rossen LM, Simon AE, Herrick KA. Types of Infant Formulas Consumed in the United States. Clin Pediatr (Phila). 2016 Mar;55(3): 278–85.
- 44 Sherman AL, Anderson J, Rudolph CD, Walker LS. Lactose-Free Milk or Soy-Based Formulas Do Not Improve Caregivers’ Distress or Perceptions of Difficult Infant Behavior. J Pediatr Gastroenterol Nutr. 2015 Jul; 61(1):119–24.
- 45 Uy N, Graf L, Lemley KV, Kaskel F. Effects of gluten-free, dairy-free diet on childhood nephrotic syndrome and gut microbiota. Pediatr Res. 2015 Jan;77(1-2):252–5.
- 46 Slupsky CM, He X, Hernell O, Andersson Y, Rudolph C, Lönnerdal B, et al. Postprandial metabolic response of breast-fed infants and infants fed lactose-free vs regular infant formula: A randomized controlled trial. Sci Rep. 2017 Jun;7(1):3640.
- 47 Agostoni C, Berni Canani R, Fairweather-Ta- it S, Heinonen M, Korhonen H, La Vieille S, et al. Scientific opinion on dietary reference values for calcium. EFSA J. 2015;13(5):4101.