Iron and Cognitive Development: What is the Evidence?
Iron and Cognitive Development: What Is the Evidence?
Leila M. Larson, Kamija S. Phiri, Sant-Rayn Pasrichac
Key Messages
- Improvement in cognitive development in children is a key rationale for universal iron interventions in pregnancy and in children.
- Data supporting effects of universal iron interventions given either antenatally or in young children on cognitive development remain ambiguous, mainly because high-quality randomized controlled trials have not yet been done.
- Treatment of anemia with oral iron in primary-school-aged children and treatment of iron deficiency anemia with parenteral iron in young children induce marked benefits on cognitive performance, indicating that effects on cognitive performance in young children are plausible.
Keywords
Iron deficiency · Cognitive development · Children · Infants · Pregnancy · Anemia · Motor development · Evidence
Abstract
The theoretical irreversible damage that iron deficiency and iron deficiency anemia can exert on child development makes a compelling argument for action to alleviate the burden. However, a critical analysis of evidence from iron interventions in early life is necessary to determine whether and how iron interventions improve cognitive outcomes. Key iron interventions used in clinical and public health practice include oral iron supplementation and, in young children, iron-containing multiple micronutrient powders. This article examines the evidence to answer 4 main questions. (1) Does antenatal iron supplementation influence long-term child cognitive development? (2) Does oral iron supplementation in preschool children improve short-term cognitive development? (3) Does oral iron supplementation in older children improve cognitive development? And (4), can provision of iron harm cognitive development? Early trials indicated benefit from parenteral iron in young children regardless of anemia status. There also appears to be evidence for benefit using oral iron treatment on cognitive performance in anemic primary school children. However, antenatal and early childhood oral iron intervention studies show inconsistent effects on early and long-term childhood cognitive outcomes. These data suggest either that (a) effects from oral iron on cognitive development in young children are small or nonexistent or that (b) heterogeneity between trials and the low quality of many studies make assessment of effect difficult. Importantly, few large, placebo-controlled trials in under-2-year-old children in low-income settings assessing effects of iron interventions on cognition have been performed; high-quality, placebo-controlled, adequately powered trials of universal iron interventions on cognitive performance in young children are urgently needed to justify policies of universal iron intervention in this group.
Introduction
Anemia affects almost 300 million children worldwide [1], and iron deficiency (ID) has been considered the most important cause. Impairments in cognitive development caused by ID comprise perhaps the key rationale for alleviating the burden of anemia in children, and an important justification for preventing anemia in pregnancy. International and national guidelines frequently recognize the importance of anemia in these groups and increasing attention is being paid to identifying resources to address it [2]. An association between impaired cognitive development and ID has long been recognized, and has underpinned the rationale for policy [3]. However, recent evidence synthesis has drawn attention to the limitations in our understanding of this topic.
There is increasing recognition of the critical importance of the first 1,000 days of a child’s existence (from conception to 2 years of age) on brain development. Hypothesized interactions between nutritional status and child development (e.g., Prado and Dewey [83]) may be direct or indirect ( Fig. 1 ). Whether and how these interactions exist for iron status remains uncertain. However, there has been enormous interest in optimizing iron stores during this sensitive period of rapid brain development [4] , and interventions which prevent or treat iron deficiency anemia (IDA) in pregnancy or in the first 2 years of life have been considered crucial to improving long-term outcomes in children [5] .
Types of Evidence
What Do We Mean by “Evidence”?
Information that can be used to inform clinical or public health policy should be derived from high-quality, robust study designs. Evidence to justify an intervention can be classified according to well-established systems. For example, the GRADE (Grading of Recommendations Assessment, Development and Evaluation) working group have established criteria to score the quality of information used to develop clinical recommendations. These systems use an explicit system to score the evidence based on study design limitations, inconsistency of results between studies, indirectness of evidence, imprecision, and reporting bias [6–8] . In most cases, only information from randomized controlled trials or systematic reviews can be considered as sufficient to provide strong recommendations to implement an intervention (Fig. 2) [6] .
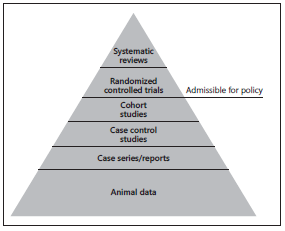
Fig. 1. Hierarchy of evidence.
Animal Studies
Animal studies are appropriate for the determination of mechanisms but inadequate for discerning a clinically relevant effect in humans. As such, animal studies are not considered as “evidence.” Experimental evidence from animal studies suggests that fetal or neonatal ID impairs brain and especially hippocampal development [9] . Early ID has also been associated with defects in myelination and impairments in dopaminergic neurotransmission [10]. Effects of ID on development of these systems have been hypothesized to produce irreversible damage to cognitive performance.
Observational Studies
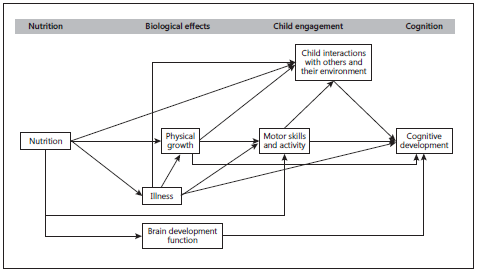
Fig. 2. Hypothetical framework for associations between nutrition and child development. Adapted from Brown and Pollitt [82] and Prado and Dewey [83] .
Observational studies start with a “low”-quality rating in GRADE. Several dozen observational studies have assessed associations between iron status and anemia in pregnancy or in infancy and compared these to cognitive outcomes in later childhood or even adulthood [11, 12] . The most important limitation of observational studies is confounding. Anemia often corresponds with impaired socioeconomic status, overall nutrition, and food security [13]; stunting [14] ; inflammation and infection (including malaria infection) [15] , exposure to indoor smoke [14], birth order, and psychosocial stimulation [16] . ID may also be associated with other confounders such as increased lead absorption [17] . Anemic or iron-deficient mothers may be less able to provide appropriate stimulation to their infants [18] . Anemic mothers may be at higher risk of depression, which may impair mother-child interactions, impairing language and cognitive development. Whilst many studies make great efforts to adjust for these confounders in their analysis, it is implausible that all such confounders are completely removed. In addition, reverse causality in observational studies may also explain some associations: for instance, more developmentally advanced children may engage more in self-feeding, or be provided with a greater iron-rich diet because they appear more mature. Indirect explanations for relationships between ID and cognition include maternal depression, which can lead to poor mother-child interaction and disrupted infant attachment [19, 20].
Randomized Controlled Trials
Double-blinded randomized controlled trials are the only way to definitively identify an effect of an intervention on an outcome. These studies provide information about the effect of an intervention on an outcome, rather than the effect of an exposure on an outcome, although analysis of effects of interventions can be substratified by exposure. Given the subjective methods for assessing child development outcomes (e.g., based on direct assessment of a child using a series of standardized tools, assessing milestones, or asking parents about perceptions of their child’s development) [21] , adequate blinding of both participants and their parents, as well as outcome assessors, is essential to generate high-quality evidence. Randomized controlled trials can be systematically identified and data combined using a formal approach (“systematic review and meta-analysis”).
From the perspective of making recommendations for interventions, data from clinical trials in humans are essential. Here, we seek to critically evaluate the evidence for effects of iron interventions in (i) pregnancy, (ii) preschool children, and (iii) older children in improving child development, with a view to understanding the potential benefits of these interventions on child development.
Does Antenatal Iron Supplementation Influence Long-Term Child Cognitive Development?
Several randomized controlled trials have evaluated effects of iron supplementation in pregnant mothers on developmental outcomes in children. Christian et al. [15] followed up a cohort of children who had been born to mothers randomized to iron-folic acid (IFA) supplementation, IFA and zinc supplementation, iron-containing multiple micronutrients (MMNs), or control (all groups also received vitamin A) during pregnancy, and in whom the prevalence of anemia and ID had been high. At 7–9 years of age, compared to children of mothers who received control, children of mothers randomized to IFA, but not of mothers who received IFA with zinc or MMNs, had higher universal nonverbal intelligence test (UNIT) results (mean in control group 48.2, mean in IFA group 51.7, adjusted mean difference 2.38, p = 0.04), superior executive function tests, and better motor function [15] . The authors had noted an attenuation by zinc of the benefits of IFA on birth weight in the original trial and hypothesized that the addition of zinc may impair iron absorption and hence the effectiveness of iron.
A smaller trial in Australia compared cognitive performance at 4 years of age in children of mothers randomized to iron or placebo during pregnancy, where IDA at delivery was uncommon (11% in the control arm) [22] . Cognitive performance was identical between groups. However, more children in the iron group had an abnormal behavior score (16%) than in the control group (8%) [23]. The authors subsequently followed the children to early school age (6–8 years of age) and again assessed behavior. Whilst there were no differences in average parent- or teacher-reported behavioral or temperament scores, there was a higher incidence of teacher-rated peer problems in the iron group (8%) than in the placebo group (2%) (p = 0.026) [24].
A randomized controlled trial in Western China randomized 5,828 pregnant women to either IFA, MMNs, or folic acid alone, and assessed 1,305 infants using the Bayley Scales at 3, 6, and 12 months of age. There were no differences between children at 3 and 6 months, but at 12 months, children of mothers who had received MMNs had a higher mental development index score by 1.22 (compared with IFA) and 1.00 (compared with folic acid alone) (p = 0.02). There were no effects on motor development [25].
In contrast, a trial of antenatal MMNs compared to either daily or twice-weekly IFA in Vietnamese women found that at the age of 6 months, children born to mothers in the twice-weekly IFA group had a higher Bayley cognitive score (mean difference 1.89) than children born to the daily IFA group (p = 0.03), despite maternal ferritin levels being lower in this group [26] . No significant differences were found compared to the MMN group.
Data from these trials were not included in Cochrane reviews or other key systematic reviews evaluating effects of iron supplementation in pregnancy [27, 28] or the United States Preventative Health Taskforce (USPSTF) review of iron supplementation in pregnancy [29] , although data from the Chinese study described above [30] were included in a Cochrane systematic review evaluating effects of antenatal MMNs [31] . Collectively, the clinical evidence does not appear to show that iron supplementation in pregnancy causes improvements in long-term child mental development.
Does Supplementation in Preschool Children Improve Short-Term Cognitive Development?
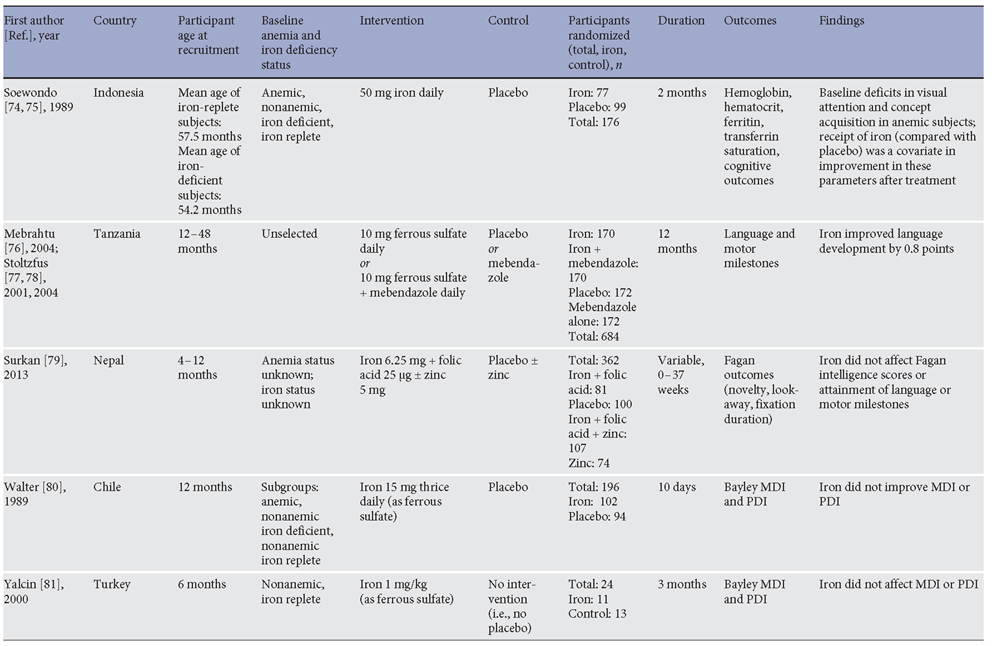
A critical rationale for preventing anemia in preschool children, and especially in children under 2 years, is the improvement of short- and long-term cognitive development [3, 32]. Several randomized controlled trials have addressed the effect of iron interventions, when compared to control, placebo, or no intervention, on cognitive development in children. Trials in children aged 6 months to 5 years evaluating effects of oral iron supplements or iron-containing multiple micronutrient powders (MNPs) on cognitive development are presented in Table 1 . Several of the key trials will be discussed here.
Two early studies evaluated effects of parenteral (intramuscular) iron treatment of IDA [33] and nonanemic ID [34] on cognitive development (assessed with the Bay-ley Mental Development Index [MDI]). Within 8 days of treatment, compared with the placebo group, children treated with IDA showed marked improvements in MDI (mean gain 13.6 points), were found to become more alert and responsive, and had improved gross and fine motor coordination [33] . Among nonanemic ID children, iron improved MDI by 21.6 points, whereas in a control group of non-ID children, there was no benefit from iron [34]. These data provided early evidence that iron interventions improve cognitive performance in iron-deficient infants.
Table 1. Randomized controlled trials comparing postnatal oral iron interventions (against control) on cognitive performance in preschool children
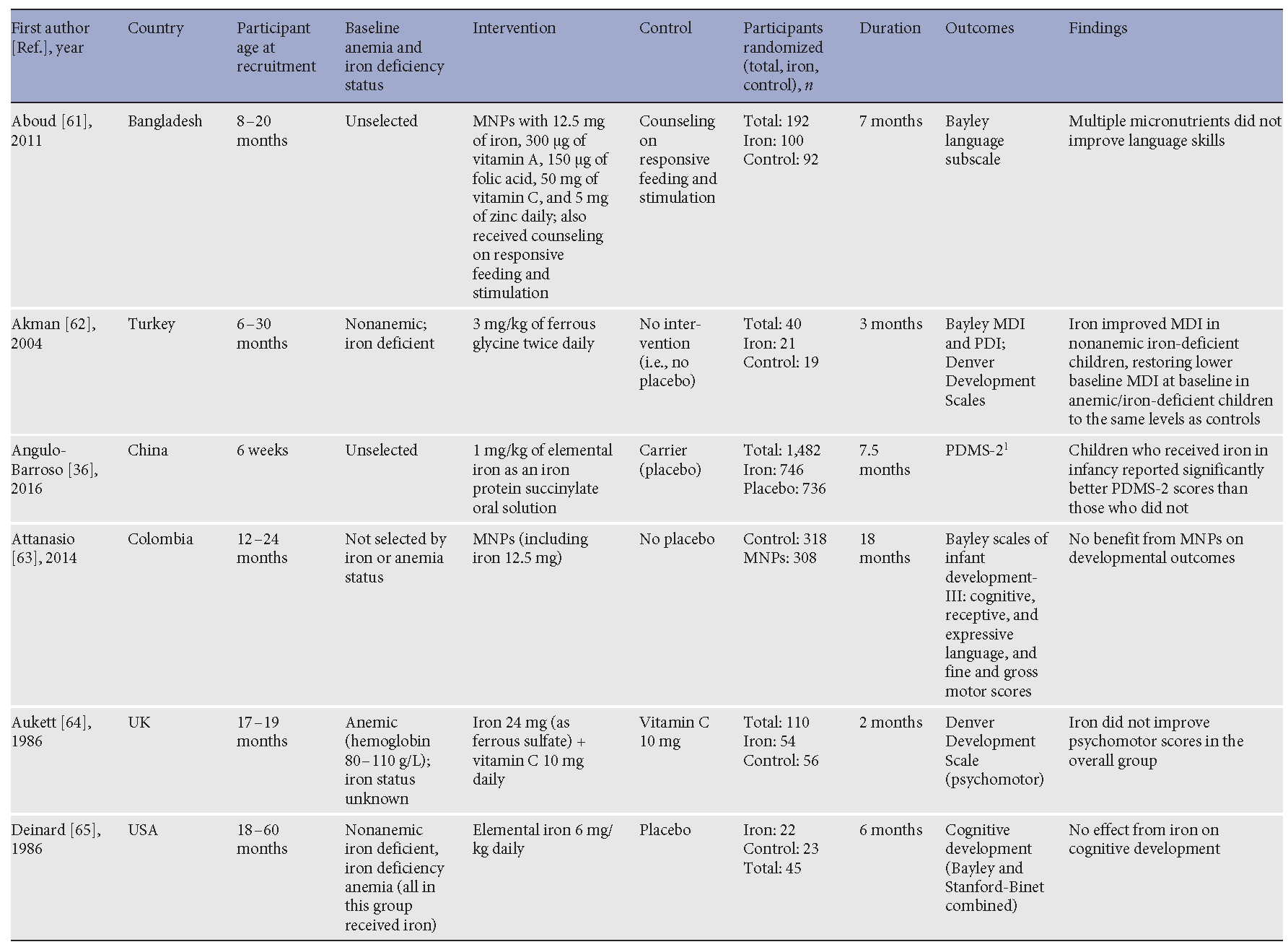
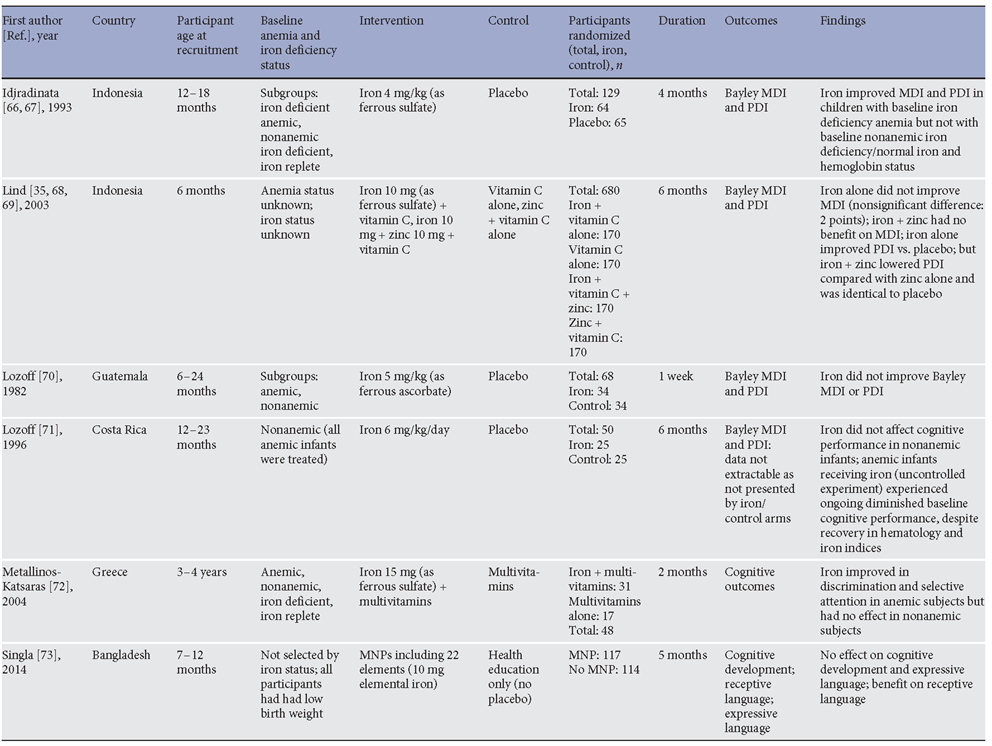
A population-based trial in 6-month-old infants was conducted in Indonesia, where 680 six-month-old infants were randomized to iron, zinc, iron and zinc, or placebo for 6 months. The trial found no benefit on mental development from iron or iron with zinc, compared to either placebo or zinc alone. There was a 3-point benefit from iron, but not iron with zinc, on motor development [35] . More recently, a large randomized trial in China comparing effects of prolonged iron supplementation to placebo in young infants (from the age of 6 weeks to 9 months) found that iron improved motor development. Effects on cognitive development, although included as an outcome in the trial registration, were not reported in this publication [36].
A 2 × 2 factorial trial in Pakistan evaluated the effectiveness of (i) responsive stimulation (local health worker delivered messages to mothers about responsive care for development, age-appropriate play activities, and problem solving), and (ii) enhanced nutrition, comprising training of health workers to deliver improved nutritional advice to mothers together with MNPs [37] . Importantly, no placebo was used in this trial. Interventions were given to infants enrolled from birth to 23 months of age (MNPs were delivered starting at 6 months of age only). Children receiving enhanced nutrition (education and MNPs) had improved cognitive, language, and socio-emotional development at 12 months and improved language (but not other parameters) at 24 months; by the time the children turned 4 years, prosocial behaviors and motor development (which had not been affected by enhanced nutrition at 24 months) were higher in the enhanced nutrition group, whereas effects on cognitive development were not seen [38] . Importantly, there were no effects from enhanced nutrition on hemoglobin concentration, and adherence to the intervention was limited [37] . Thus, this trial may have identified a transient early improvement in cognitive development which was not sustained throughout the intervention period, and the mechanism may not have been mediated directly via the effect of contents of the MNPs on anemia.
In slightly older children, Stoltzfus et al. [39] randomized 614 Tanzanian preschool children (6–59 months) to 12 months of iron supplementation (10 mg), antihelminthic therapy, a combination of the two, or placebo. The mean age at baseline was about 30 months. Motor and language milestones were assessed by parental report. In this group, nearly all children were anemic at baseline. Iron was found to improve motor scores in children who had been most anemic at baseline, but not overall. Iron improved language scores by 0.8 points on average (p = 0.011) [39] . The placebo-controlled design of the study meant the authors were able to conclude that iron improved child development.
Several trials have attempted to address the effects of iron interventions in children younger than 6 months, either by fortifying infant formula or giving iron supplements to very young children. For example, a small trial gave oral iron supplements or placebo to term breastfed infants from 1 to 6 months of age, and found improvements from iron in visual acuity and psychomotor development but not mental development at 13 months of age [40] . Likewise, no improvements in mental development were reported in a trial comparing iron-fortified with low-iron formula in Canadian children from very low-income households; benefits on motor development were seen at 9 and 12 months of
age, but were attenuated by 15 months [41] .
Systematic reviews have summarized effects of iron supplementation on child cognitive development through meta-analysis. Pasricha et al. [42] meta-analyzed the effects of daily iron supplementation in children 4–23 months of age and found no clear evidence of benefit from iron on cognitive development at the end of the intervention (mean difference 1.65 [95% CI –0.63, 3.94], p = 0.16; 6 trials). Even when only studies at low risk of bias were included, the effect size was nonsignificant. Likewise, no benefit from iron was observed among children who had been anemic at baseline [42] . Thompson et al. [43] assessed effects of daily iron supplementation in 2- to 5-year-old children, and identified 4 trials including Stoltzfus described above, along with 2 other trials that did not clearly report overall effect sizes from iron on cognitive development. A previous review of the effect of iron interventions in children (delivered through any mechanism) likewise was unable to identify a beneficial effect from iron on cognitive development in children under 27 months of age, although a benefit in older children was seen [44] . Wang et al. [45] undertook a Cochrane review to assess effects of iron supplementation on cognitive development in iron-deficient anemic children under 3 years and did not find evidence that iron supplementation improved cognitive development (1.04 [–1.30, 3.39], p = 0.79) in this group. Larson and Yousafzai [46] meta-analyzed the effects of various nutritional supplementation regimens given postnatally to infants and children on cognitive development (combining trials giving iron, zinc, or MMN combinations of these) in low- and middle-income countries and reported that these did not significantly improve child development. Finally, the USPSTF analysis of evidence for iron interventions in high-income countries identified 4 randomized controlled trials, but was unable to find evidence of a benefit from iron on cognitive development [47] . Thus, evidence synthesis approaches have failed to identify evidence of benefit from iron supplementation on cognitive development in children under 2 years of age.
Perhaps more important than short-term cognitive performance are the effects of iron on long-term cognitive development. Two studies have evaluated the effects of iron interventions in the first 2 years of life on longterm cognitive outcomes. Murray-Kolb et al. [48] followed up a cohort of 735 Nepalese children who had been given daily doses of IFA, zinc, IFA with zinc, or placebo at the age of 12–35 months for a variable duration depending on age of enrolment. The children were assessed at the age of 7–9 years using tests of cognitive, executive, and motor function. The authors found that compared with children who had received placebo, there were no differences in children who had received IFA (with or without zinc) either before or after adjustment for con-founders. In the adjusted analysis, children who were given IFA supplementation from 12 to 18 months of age (i.e., for the longest duration) performed worse (p = 0.02) on tests of executive function and fine motor function compared with children who did not receive IFA [48] . A second trial, in Thailand, followed up 560 of 609 children originally randomized at the age of 6 months to iron (10 mg), zinc, iron and zinc, or placebo given daily for 6 months. Children were followed up at the age of 9 years, when the authors found no differences overall or in any domain of the Wechsler Intelligence Scale for Children-III between the groups, or in the Raven’s Colored Progressive Matrices score. Moreover, no differences in school performance in any subject (Thai, English, Mathematics, or Science) were observed [49] . Collectively, these studies do not support the hypothesis that even quite sustained courses of iron supplementation in young children benefit cognitive performance in the long term.
Failure to convincingly identify a benefit from iron on cognition may be because such a benefit does not exist – perhaps because iron at these doses and via this route does not impact on brain development, or, as hypothesized by some authors, because the effects from ID on early brain development are irreversible [50, 51] . However, the “irreversible damage” hypothesis is not supported by the improvements seen in early trials of parenteral iron in iron-deficient infants [33, 34] . Alternatively, studies have been undertaken in heterogeneous populations or populations where effects from iron may be unlikely to produce benefit. Several of the trials included in Table 1 were very short (<2 weeks) in duration. Several earlier, smaller trials conducted with small samples, using niche cognitive tests, and reported in ways that do not disclose the overall effect size from iron are perhaps better considered as clinical experiments rather than as formal randomized controlled trials designed to generate evidence.
Interactions between Cognitive and Psychomotor Development
Although meta-analyses do not clearly demonstrate benefits from iron on psychomotor development (including gross and fine motor skills), several of the studies investigating effects of iron in preschool-aged children identified benefits on psychomotor (rather than cognitive) development [35, 36, 52] . It is possible that benefits on psychomo-tor development may indirectly result in improved cognitive development. Using structural equation modeling, observational studies in Indonesia and Tanzania considered that associations between nutrition and mental development were mediated by motor activity and motor development [53, 54] . Possible effects of benefits of motor development on mental development may be explained by the child having richer experiences, more stimulating situations, and improved interactions with others [55] .
Does Iron Supplementation in Older Children Improve Cognitive Development?
Several trials have evaluated effects of iron supplementation in primary schoolchildren, and Low et al. [56] summarized these studies in a recent systematic review. The authors identified 9 studies conducted in primary school children aged 5–12 years, measuring cognitive testing. Intelligence quotient (IQ) was measured in 5 studies, whilst 3 studies used other author-adapted scales of cognitive performance, and 1 used school performance. Overall, compared with control, iron improved global cognitive scores (standardized mean difference 0.50 [0.11, 0.90], p = 0.01; 9 studies). This benefit was only seen among children who had been anemic at baseline (standardized mean difference 0.29 [0.07, 0.51], p = 0.01; 6 studies), but not in children who had been nonanemic at enrolment. There was no overall benefit from iron supplements in IQ in primary school children (mean difference 5.47 [–3.24, 4.18], p = 0.2; 5 studies), although among anemic children, iron supplements did improve IQ (mean difference
4.55 [0.16, 8.94], p = 0.04; 3 studies). Thus, in contrast to trials undertaken in preschool children, treatment of anemia with iron supplementation in older children demonstrates a benefit on cognitive performance.
These data are corroborated by other systematic reviews. Sachdev et al. [44] found a beneficial effect from iron interventions (oral or parenteral iron supplementation, fortified formula milk or cereals) on IQ in children aged 8 years or older (standardized mean difference 0.41 [0.20, 0.62], p < 0.001) [45] . Likewise, Falkingham et al.
[57] summarized 14 trials evaluating the effects of iron supplementation in older children, adolescents, and women on cognitive performance and found that iron improved IQ by 2.5 points (1.24, 3.76), although it had no effect in nonanemic individuals or on other outcomes including academic achievement, memory, or motor development [57] .
Can Provision of Iron Harm Cognitive Development?
There have been emerging concerns that provision of high concentrations of iron in fortification infant formula may impair long-term cognitive development. Lozoff et al. [58] followed up a cohort of Chilean infants who had been randomized in infancy to receive infant formula fortified with either low (2.3 mg/L) or high (12.7 mg/L) levels of iron. After 6 months, children given the high-iron-fortified formula and/or iron supplements did not exhibit improved cognitive or psychomotor development, but had approximately 5 days earlier age of crawling and had a delayed Fagan looking time, which the authors thought would predict impaired longer-term cognitive performance [58] . However, at the age of 10 years, the authors reassessed the children and found that those who had been randomized to the high-iron formula performed more poorly than their low-formula-receiving counterparts on every outcome (IQ, p = 0.06;spatial memory, p = 0.02; arithmetic achievement, p = 0.07; visual-motor integration, p = 0.046; visual perception, p = 0.06) [59] . A recent update to this cohort indicated a similar pattern of adverse performance in the high-iron-fortified group at the age of 16 years; of 9 cognitive tests performed, 4 showed poorer performance in the high-iron group; baseline hemoglobin did not interact with these effects [60].
Conclusions
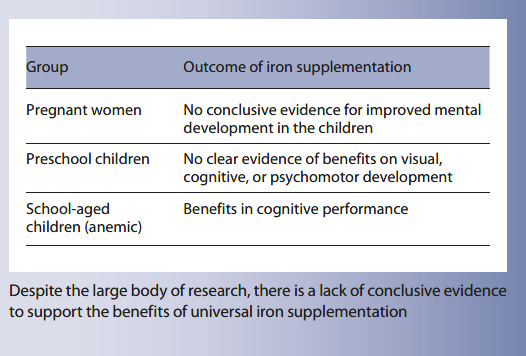
Improvements in long-term child development are perhaps the most important justification for universal iron (iron supplement or MNPs) interventions in young children and considered an important reason for preventing anemia in pregnancy. Whilst there is clear evidence for benefits of iron treatment on cognitive performance in anemic primary school children, in younger children, and especially in children under 2 years of age, evidence that oral iron interventions (supplements or mi-cronutrient powders) improve short- and long-term cognitive development remains scant. Benefits from parenteral iron on cognition in iron-deficient and anemic infants have been reported in early trials and refute the contention that iron-induced brain damage is intractable, instead suggesting either that oral iron is ineffective or that quality trials have not yet been performed. Likewise, effects of iron in pregnancy on early and longer-term cognitive development remain ambiguous, with failure to demonstrate consistency or dose response. Heterogeneity may be due to differences in dose and duration of iron, timing of commencement of iron, population and epidemiology of anemia and ID, and adherence to interventions.
Although numerous studies purporting to address these questions are available, the issue has still not been addressed in high-quality randomized controlled trials. Several recent trials evaluating effects of iron interventions (including MNPs) were not placebo controlled. Given the potentially subjective nature of cognitive assessments, blinding is essential, and non-placebo-controlled studies do not provide useful evidence for this outcome, regardless of the direction of the finding. We strongly discourage investigators from undertaking non-placebo-controlled trials in the future as they will not add to the literature. High-quality, dedicated, placebo-controlled, adequately powered trials of universal iron interventions on cognitive performance in young children are urgently needed. In the interim, improvements in cognitive development cannot be used as a rationale for implementing universal iron interventions.
Disclosure Statement
The authors have no conflicts of interest to declare. The writing of this article was supported by Nestlé Nutrition Institute.
References
- World Health Organization: The Global Prevalence of Anaemia in 2011. Geneva, World Health Organization, 2015.
- World Health Organization: Essential Nutrition Actions. Geneva, World Health Organization, 2013.
- WHO/UNICEF/UNU: Iron Deficiency Anaemia: Assessment, Prevention, and Control. A Guide for Programme Managers. Geneva, World Health Organization, 2001.
- Cusick SE, Georgieff MK: The role of nutrition in brain development: the golden opportunity of the “first 1,000 days.” J Pediatr 2016; 175: 16–21.
- 1,000 Days: 1,000 Days. Washington, DC, 1,000 Days, 2017.
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, Norris S, Falck-Ytter Y, Glasziou P, Jaeschke R: GRADE guidelines: 1. Introduction – GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011; 64: 383–394.
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schunemann HJ: What is “quality of evidence” and why is it important to clinicians? BMJ 2008; 336: 995–998.
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schunemann HJ: GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008; 336: 924–926.
- Jorgenson LA, Wobken JD, Georgieff MK: Perinatal iron deficiency alters apical den-dritic growth in hippocampal CA1 pyramidal neurons. Dev Neurosci 2003; 25: 412–420.
- Doom JR, Georgieff MK: Striking while the iron is hot: Understanding the biological and neurodevelopmental effects of iron deficiency to optimize intervention in early childhood. Curr Pediatr Rep 2014; 2: 291–298.
- McCann JC, Ames BN: An overview of evidence for a causal relation between iron deficiency during development and deficits in cognitive or behavioral function. Am J Clin Nutr 2007; 85: 931–945.
- Grantham-McGregor S, Ani C: A review of studies on the effect of iron deficiency on cognitive development in children. J Nutr 2001; 131:649S–666S; discussion 666S–668S.
- Pasricha SR, Black J, Muthayya S, Shet A, Bhat V, Nagaraj S, Prashanth NS, Sudarshan H, Biggs BA, Shet AS: Determinants of anemia among young children in rural India. Pediatrics 2010; 126:e140–e149.
- Mishra V, Retherford RD: Does biofuel smoke contribute to anaemia and stunting in early childhood? Int J Epidemiol 2007; 36: 117–129.
- Christian P, Murray-Kolb LE, Khatry SK, Katz J, Schaefer BA, Cole PM, Leclerq SC, Tielsch JM: Prenatal micronutrient supplementation and intellectual and motor function in early school-aged children in Nepal. JAMA 2010; 304: 2716–2723.
- Tofail F, Hamadani JD, Mehrin F, Ridout DA, Huda SN, Grantham-McGregor SM: Psychosocial stimulation benefits development in nonanemic children but not in anemic, iron-deficient children. J Nutr 2013; 143: 885–893.
- Wolf AW, Jimenez E, Lozoff B: Effects of iron therapy on infant blood lead levels. J Pediatr 2003; 143: 789–795.
- Perez EM, Hendricks MK, Beard JL, Murray-Kolb LE, Berg A, Tomlinson M, Irlam J, Isaacs W, Njengele T, Sive A, Vernon-Feagans L: Mother-infant interactions and infant development are altered by maternal iron deficiency anemia. J Nutr 2005; 135: 850–855.
- Aboud FE, Singla DR, Nahil MI, Borisova I: Effectiveness of a parenting program in Bangladesh to address early childhood health, growth and development. Soc Sci Med 2013; 97: 250–258.
- Black MM, Baqui AH, Zaman K, McNary SW, Le K, Arifeen SE, Hamadani JD, Parveen M, Yunus M, Black RE: Depressive symptoms among rural Bangladeshi mothers: implications for infant development. J Child Psychol Psychiatry 2007; 48: 764–772.
- Bedford H, Walton S, Ahn J: Measures of Child Development – A Review. London, Centre for Paediatric Epidemiology and Biostatistics, UCL Institute of Child Health, 2013.
- Makrides M, Crowther CA, Gibson RA, Gibson RS, Skeaff CM: Efficacy and tolerability of low-dose iron supplements during pregnancy: a randomized controlled trial. Am J Clin Nutr 2003; 78: 145–153.
- Zhou SJ, Gibson RA, Crowther CA, Baghurst P, Makrides M: Effect of iron supplementation during pregnancy on the intelligence quotient and behavior of children at 4 y of age: long-term follow-up of a randomized controlled trial. Am J Clin Nutr 2006; 83: 1112–1117.
- Parsons AG, Zhou SJ, Spurrier NJ, Makrides
M:Effect of iron supplementation during pregnancy on the behaviour of children at early school age: long-term follow-up of a randomised controlled trial. Br J Nutr 2008; 99: 1133–1139. - Li Q, Yan H, Zeng L, Cheng Y, Liang W, Dang S, Wang Q, Tsuji I: Effects of maternal multimicronutrient supplementation on the mental development of infants in rural western China: follow-up evaluation of a double-blind, randomized, controlled trial. Pediatrics 2009; 123:e685–e692.
- Hanieh S, Ha TT, Simpson JA, Casey GJ, Khuong NC, Thoang DD, Thuy TT, Pasricha SR, Tran TD, Tuan T, Dwyer T, Fisher J, Biggs BA: The effect of intermittent antenatal iron supplementation on maternal and infant outcomes in rural Viet Nam: a cluster randomised trial. PLoS Med 2013; 10: e1001470.
- Haider BA, Olofin I, Wang M, Spiegelman D, Ezzati M, Fawzi WW; Nutrition Impact Model Study Group: Anaemia, prenatal iron use, and risk of adverse pregnancy outcomes: systematic review and meta-analysis. BMJ 2013; 346:f3443.
- Pena-Rosas JP, De-Regil LM, Garcia-Casal MN, Dowswell T: Daily oral iron supplementation during pregnancy. Cochrane Database Syst Rev 2015;CD004736.
- McDonagh M, Cantor A, Bougatsos C, Dana T, Blazina I: Routine Iron Supplementation and Screening for Iron Deficiency Anemia in Pregnant Women: A Systematic Review to Update the U.S. Preventive Services Task Force Recommendation. Evidence Synthesis No. 123. Rockville, MD, 2015.
- Zeng L, Dibley MJ, Cheng Y, Dang S, Chang S, Kong L, Yan H: Impact of micronutrient supplementation during pregnancy on birth weight, duration of gestation, and perinatal mortality in rural western China: double blind cluster randomised controlled trial. BMJ 2008; 337:a2001.
- Haider BA, Bhutta ZA: Multiple-micronutrient supplementation for women during pregnancy. Cochrane Database Syst Rev 2017; 4:CD004905.
- Black MM, Quigg AM, Hurley KM, Pepper MR: Iron deficiency and iron-deficiency anemia in the first two years of life: strategies to prevent loss of developmental potential. Nutr Rev 2011; 69(suppl 1):S64–S70.
- Oski FA, Honig AS: The effects of therapy on the developmental scores of iron-deficient infants. J Pediatr 1978; 92: 21–25.
- Oski FA, Honig AS, Helu B, Howanitz P: Effect of iron therapy on behavior performance in nonanemic, iron-deficient infants. Pediatrics 1983; 71: 877–880.
- Lind T, Lonnerdal B, Stenlund H, Gamayan-ti IL, Ismail D, Seswandhana R, Persson LA: A community-based randomized controlled trial of iron and zinc supplementation in Indonesian infants: effects on growth and development. Am J Clin Nutr 2004; 80: 729– 736.
- Angulo-Barroso RM, Li M, Santos DC, Bian Y, Sturza J, Jiang Y, Kaciroti N, Richards B, Lozoff B: Iron supplementation in pregnancy or infancy and motor development: a randomized controlled trial. Pediatrics 2016; 137.
- Yousafzai AK, Rasheed MA, Rizvi A, Armstrong R, Bhutta ZA: Effect of integrated responsive stimulation and nutrition interventions in the Lady Health Worker programme in Pakistan on child development, growth, and health outcomes: a cluster-randomised factorial effectiveness trial. Lancet 2014; 384: 1282–1293.
- Yousafzai AK, Obradovic J, Rasheed MA, Rizvi A, Portilla XA, Tirado-Strayer N, Siyal S, Memon U: Effects of responsive stimulation and nutrition interventions on children’s development and growth at age 4 years in a disadvantaged population in Pakistan: a longitudinal follow-up of a cluster-randomised factorial effectiveness trial. Lancet Glob Health 2016; 4:e548–e558.
- Stoltzfus RJ, Kvalsvig JD, Chwaya HM, Mon-tresor A, Albonico M, Tielsch JM, Savioli L, Pollitt E: Effects of iron supplementation and anthelmintic treatment on motor and language development of preschool children in Zanzibar: double blind, placebo controlled study. Br Med J 2001; 323: 1389–1393.
- Friel JK, Aziz K, Andrews WL, Harding SV, Courage ML, Adams RJ: A double-masked, randomized control trial of iron supplementation in early infancy in healthy term breastfed infants. J Pediatr 2003; 143: 582–586.
- Moffatt ME, Longstaffe S, Besant J, Dureski
C:Prevention of iron deficiency and psycho-motor decline in high-risk infants through use of iron-fortified infant formula: a randomized clinical trial. J Pediatr 1994; 125: 527–534. - Pasricha S-R, Hayes E, Kalumba K, Biggs B-A: Effect of daily iron supplementation on health in children aged 4–23 months: a systematic review and meta-analysis of ran-domised controlled trials. Lancet Glob Health 2013; 1:e77–e86.
- Thompson J, Biggs BA, Pasricha SR: Effects of daily iron supplementation in 2- to 5-year-old children: systematic review and meta-analysis. Pediatrics 2013; 131: 739–753.
- Sachdev H, Gera T, Nestel P: Effect of iron supplementation on mental and motor development in children: systematic review of randomised controlled trials. Public Health Nutr 2005; 8: 117–132.
- Wang B, Zhan S, Gong T, Lee L: Iron therapy for improving psychomotor development and cognitive function in children under the age of three with iron deficiency anaemia. Cochrane Database Syst Rev 2013; 6: CD001444.
- Larson LM, Yousafzai AK: A meta-analysis of nutrition interventions on mental development of children under-two in low- and middle-income countries. Matern Child Nutr 2017; 13.
- McDonagh M, Blazina I, Dana T, Cantor A, Bougatsos C: Routine Iron Supplementation and Screening for Iron Deficiency Anemia in Children Ages 6 to 24 Months: A Systematic Review to Update the U.S. Preventive Services Task Force Recommendation. Rockville, MD, 2015.
- Murray-Kolb LE, Khatry SK, Katz J, Schaefer BA, Cole PM, LeClerq SC, Morgan ME, Tielsch JM, Christian P: Preschool micronutrient supplementation effects on intellectual and motor function in school-aged Nepalese children. Arch Pediatr Adolesc Med 2012; 166: 404–410.
- Pongcharoen T, DiGirolamo AM, Ramakrishnan U, Winichagoon P, Flores R, Mar-torell R: Long-term effects of iron and zinc supplementation during infancy on cognitive function at 9 y of age in northeast Thai children: a follow-up study. Am J Clin Nutr 2011; 93: 636–643.
- Nelson CA, Wewerka S, Thomas KM, deRegnier R-A, Tribbey-Walbridge S, Georgieff
M:Neurocognitive sequelae of infants of diabetic mothers. Behav Neurosci 2000; 114: 950. - Bauer PJ: Declarative memory in infancy: an introduction to typical and atypical development. Adv Child Dev Behav 2010; 38: 1–25.
- Olney DK, Pollitt E, Kariger PK, Khalfan SS, Ali NS, Tielsch JM, Sazawal S, Black R, Allen LH, Stoltzfus RJ: Combined iron and folic acid supplementation with or without zinc reduces time to walking unassisted among Zanzibari infants 5- to 11-mo old. J Nutr 2006; 136: 2427–2434.
- Olney DK, Kariger PK, Stoltzfus RJ, Khalfan SS, Ali NS, Tielsch JM, Sazawal S, Black R, Allen LH, Pollitt E: Developmental effects of micronutrient supplementation and malaria in Zanzibari children. Early Hum Dev 2013; 89: 667–674.
- Pollitt E, Jahari A, Walka H: A developmental view of the effects of an energy and micro-nutrient supplement in undernourished children in Indonesia. Eur J Clin Nutr 2000; 54(suppl 2):S107–S113.
- Adolph KE, Tamis-LeMonda CS: The costs and benefits of development: the transition from crawling to walking. Child Dev Per-spect 2014; 8: 187–192.
- Low M, Farrell A, Biggs BA, Pasricha SR: Effects of daily iron supplementation in prima-ry-school-aged children: systematic review and meta-analysis of randomized controlled trials. CMAJ 2013; 185:E791–E802.
- Falkingham M, Abdelhamid A, Curtis P, Fairweather-Tait S, Dye L, Hooper L: The effects of oral iron supplementation on cognition in older children and adults: a systematic review and meta-analysis. Nutr J 2010; 9.
- Lozoff B, De Andraca I, Castillo M, Smith JB, Walter T, Pino P: Behavioral and developmental effects of preventing iron-deficiency anemia in healthy full-term infants. Pediatrics 2003; 112: 846–854.
- Lozoff B, Castillo M, Clark KM, Smith JB: Iron-fortified vs low-iron infant formula: developmental outcome at 10 years. Arch Pediatr Adolesc Med 2012; 166: 208–215.
- Gahagan S, Delker E, Castillo M, Lozoff B: Iron Fortified vs Low-Iron Infant Formula: Cognitive Outcomes at 10 and 16 Years: Seventh Congress of the International BioIron Society (IBIS). Los Angeles, 2017.
- Aboud FE, Akhter S: A cluster-randomized evaluation of a responsive stimulation and feeding intervention in Bangladesh. Pediatrics 2011; 127:e1191–e1197.
- Akman M, Cebeci D, Okur V, Angin H, Abali O, Akman AC: The effects of iron deficiency on infants’ developmental test performance. Acta Paediatr 2004; 93: 1391–1396.
- Attanasio OP, Fernandez C, Fitzsimons EO, Grantham-McGregor SM, Meghir C, Rubio-Codina M: Using the infrastructure of a conditional cash transfer program to deliver a scalable integrated early child development program in Colombia: cluster randomized controlled trial. BMJ 2014; 349:g5785.
- Aukett MA, Parks YA, Scott PH, Wharton BA: Treatment with iron increases weight gain and psychomotor development. Arch Dis Child 1986; 61: 849–857.
- Deinard AS, List A, Lindgren B, Hunt JV, Chang PN: Cognitive deficits in iron-deficient and iron-deficient anemic children. J Pediatr 1986; 108: 681–689.
- Idjradinata P, Pollitt E: Reversal of developmental delays in iron-deficient anaemic infants treated with iron. Lancet 1993; 341: 1–4.
- Idjradinata P, Watkins WE, Pollitt E: Adverse effect of iron supplementation on weight gain of iron-replete young children. Lancet 1994; 343: 1252–1254.
- Lind T, Lonnerdal B, Stenlund H, Ismail D, Seswandhana R, Ekstrom EC, Persson LA: A community-based randomized controlled trial of iron and zinc supplementation in Indonesian infants: interactions between iron and zinc. Am J Clin Nutr 2003; 77: 883–890.
- Lind T, Seswandhana R, Persson LA, Lon-nerdal B: Iron supplementation of iron-replete Indonesian infants is associated with reduced weight-for-age. Acta Paediatr 2008; 97: 770–775.
- Lozoff B, Brittenham GM, Viteri FE, Wolf AW, Urrutia JJ: The effects of short-term oral iron therapy on developmental deficits in iron-deficient anemic infants. J Pediatr 1982; 100: 351–357.
- Lozoff B, Wolf AW, Jimenez E: Iron-deficiency anemia and infant development: effects of extended oral iron therapy. J Pediatr 1996; 129: 382–389.
- Metallinos-Katsaras E, Valassi-Adam E, Dewey KG, Lonnerdal B, Stamoulakatou A, Pollitt E: Effect of iron supplementation on cognition in Greek preschoolers. Eur J Clin Nutr 2004; 58: 1532–1542.
- Singla DR, Shafique S, Zlotkin SH, Aboud FE: A 22-element micronutrient powder benefits language but not cognition in Bangladeshi full-term low-birth-weight children. J Nutr 2014; 144: 1803–1810.
- Yurdakok K, Temiz F, Yalcin SS, Gumruk F: Efficacy of daily and weekly iron supplementation on iron status in exclusively breast-fed infants. J Pediatr Hematol Oncol 2004; 26: 284–288.
- Soewondo S, Husaini M, Pollitt E: Effects of iron deficiency on attention and learning processes in preschool children: Bandung, Indonesia. Am J Clin Nutr 1989; 50: 667–673.
- Mebrahtu T, Stoltzfus RJ, Chwaya HM, Jape JK, Savioli L, Montresor A, Albonico M, Tielsch JM: Low-dose daily iron supplementation for 12 months does not increase the prevalence of malarial infection or density of parasites in young Zanzibari children. J Nutr 2004; 134: 3037–3041.
- Stoltzfus RJ, Chway HM, Montresor A, Tielsch JM, Jape JK, Albonico M, Savioli L: Low dose daily iron supplementation improves iron status and appetite but not anemia, whereas quarterly anthelminthic treatment improves growth, appetite and anemia in Zanzibari preschool children. J Nutr 2004; 134: 348–356.
- Stoltzfus RJ, Kvalsvig JD, Chwaya HM, Mon-tresor A, Albonico M, Tielsch JM, Savioli L, Pollitt E: Effects of iron supplementation and anthelmintic treatment on motor and language development of preschool children in Zanzibar: double blind, placebo controlled study. BMJ 2001; 323: 1389–1393.
- Surkan PJ, Siegel EH, Patel SA, Katz J, Khatry SK, Stoltzfus RJ, Leclerq SC, Tielsch JM: Effects of zinc and iron supplementation fail to improve motor and language milestone scores of infants and toddlers. Nutrition 2013; 29: 542–548.
- Walter T, De Andraca I, Chadud P, Perales CG: Iron deficiency anemia: adverse effects on infant psychomotor development. Pediatrics 1989; 84: 7–17.
- Yalcin SS, Yurdakok K, Acikgoz D, Ozmert
E: Short-term developmental outcome of iron prophylaxis in infants. Pediatr Int 2000; 42: 625–630. - Brown JL, Pollitt E: Malnutrition, poverty and intellectual development. Sci Am 1996; 274: 38–43.
- Prado EL, Dewey KG: Nutrition and brain development in early life. Nutr Rev 2014; 72: 267–284.