The Importance of Dietary Protein at Breakfast in Childhood
Abstract
In order for the body to maintain a healthy and normal steady state of lean body mass, body proteins constantly undergo breakdown and synthesis. The rate at which lean tissue is synthetized must equal the rate at which it is being broken down in order to maintain body protein levels. During growth, not only is there an increase in the net deposition of protein, but the rates of both protein synthesis and breakdown are also increased leading potentially to an increased demand of dietary protein in conditions of increased turnover. When referring to growth, the growth potential of an individual in height and overall shape tends to be genetically determined so that each individual follows a growth curve canalized in terms of both extent and time course if conditions are favorable, where nutrition status may be classed as such as a favorable condition. To this end, identifying specific moments throughout the day whereby whole body protein balance is in a net negative state may provide key opportunities for specific nutrient provision with the aim of optimizing whole body protein accrual.
Introduction
An important factor influencing the general health and well-being of an individual is the pattern of nutrient intake across the day. Unhealthful meal patterns have been implicated in obesity, cholesterol/lipoprotein levels, glucose metabolism, plasma hormones, caloric density to energy intake, and nutrient utilization [1]. Breakfast is often considered as one of the most important meals of the day as it is an opportunity to replenish nutrition to the body following a prolonged overnight fast. Beyond the nutrient replenishment aspect of breakfast, much work has focused on breakfast consumption and skipping behaviors and their impact on health outcomes [2], although the causality effect is not clear [3]. However, clear recommendations relating to the percentage of daily energy that should be consumed at breakfast are lacking. Furthermore, although it is widely accepted that macronutrients such as carbohydrates are key to replenishing glycogen stores after sport, very little is known regarding the contribution of protein consumption at breakfast in establishing a platform for healthy physical growth in children. To this end, the present work will focus on the physiological consequences of over- night fasting in children, and the way dietary protein intake at breakfast contributes to the recovery of whole body protein following an overnight fast.
Dietary Protein
Proteins (derived from the Greek word πρώτειος [proteios] meaning “first one” or “most important one”) are the major functional and structural components of all the cells in the body and participate in virtually all biological processes occurring in the body. This is achieved by their constituent amino acids that are used to build and maintain bones, muscles, and skin, and to produce molecules with important physiological roles, such as enzymes, hormones, neurotransmitters, and antibodies. To this end, one of the most important aspects and defining characteristics of a given dietary protein from a nutritional standpoint is its amino acid composition and the capacity of these amino acids to be digested. Beyond the importance of dietary proteins in the growth and maintenance of body tissue, they also act as signaling molecules and as a source of energy. Dietary proteins are composed of 20 amino acids. Of these, 9 are considered to be essential in that in humans, they cannot be synthesized and are, therefore, required in the diet.
One dietary source providing a nutritionally complete type of dietary protein in terms of amino acid profile and digestibility is milk. Specifically, an average glass (∼220–250 mL) of cow’s milk typically contains approximately 7 g of protein, of which approximately 20% are whey protein fractions while the remaining 80% are composed of casein protein fractions. Although both whey and casein protein fractions are classed as high-quality dietary proteins, these protein components of cow’s milk result in very important and disparate physiological responses. Following the ingestion of whey protein, for instance, the plasma appearance of dietary amino acids is fast, high, and transient [4]. On the other hand, casein ingestion results in a slower, lower, and more prolonged response in terms of plasma amino acid concentrations [4]. In addition, it has previously been reported in intervention studies carried out in healthy children that whey ingestion stimulates a greater insulin response than casein while casein ingestion results in greater levels of plasma IGF-1 [5]. Therefore, collectively, these differential physiological responses of the main milk protein fractions exhibit important but different metabolic and growth-promoting effects.
Diurnal Protein Turnover
When healthy individuals consume regular mixed meals and maintain activities of everyday living, whole body and skeletal muscle protein mass remains essentially unchanged for lengthy periods of time. This is because over time, the rates of protein synthesis approximate the rates of protein breakdown, resulting in the maintenance of whole body protein balance. In the instance where an increase in lean tissue is desired, such as in growing children, it is asserted that the net rate of protein synthesis be greater than the rate of protein breakdown, and this shift must be sustained over time in order to promote overall growth. However, this process is not static and fluctuates across a 24-h period depending on feeding patterns (including macronutrient intake) as well as physical activity duration and type. For example, feeding has been shown to almost double the whole body synthetic response in adults [6], an effect that appears mainly due to the effects of increased amino acid availability (particularly essential amino acids) rather than the feeding-induced in- crease in insulin concentrations. Evidence to support this contention comes from investigations that have “clamped” insulin levels (by the use of somatostatin and analogs) and still observed robust increases in protein synthesis [7]. Increased amino acid availability not only provides substrates for protein synthesis but also directly modulates intracellular signaling events regulating initiation and elongation phases of mRNA translation [7]. On the other hand, increased insulin availability as observed in the postprandial state following a meal containing carbohydrates has been shown to directly attenuate the rates of protein breakdown [7].
Dietary Protein in Children
Dietary protein in children is important for maintaining the growth and remodeling of tissues like bones and muscles [8]. Current recommendations for dietary protein in children do not distinguish between specific times of the day, are similar to those in healthy adults (0.92 vs. 0.83 g/kg per day, respectively), and are assumed to be adequate to ensure a positive nitrogen balance for optimal growth in all children [9]. In contrast to adults, children are in a constant growth phase with a typical growth rate of approximately 5 cm and growth weight of approximately 3 kg per year up to the age of ∼10 years of age [10].
Identifying key moments whereby dietary protein may be beneficial in children’s physical growth and development has recently been investigated. Specifically, it was recently reported that following exercise, physically active healthy children are in a state of negative whole body protein balance and that protein ingestion immediately following cessation of exercise is able to promote a positive balance in a dose-dependent manner [11]. However, it seems that more is not necessarily better. For instance, recent work suggests that evenly distributing 15 g of dietary protein 4 h apart following cessation of exercise resulted in great- er whole body protein balance over a 24-h period than 15 g immediately or 4 h after exercise cessation in children [12].
However, increased intakes of dietary protein in early childhood beyond that of the current recommendations have been associated with increased risk of childhood obesity [13, 14]. Furthermore, the source of dietary protein may also be important as studies have shown stronger associations between high animal-sourced protein intake and higher body weight compared to plant-sourced proteins with the increased weight resulting from greater adiposity [15]. In contrast, further recent work on the longitudinal association of increased dietary protein intake in children reported that higher protein intake in mid-childhood (8 years of age) was associated with increased fat-free mass at 10 years of age. Further- more, the authors also reported that protein intake from plant sources was beneficial for body composition in these school-age children [16]. The exact reasons behind these observations are not clear. However, it has been proposed to be due to the “early protein hypothesis,” which states that protein intake in early life can stimulate adipogenesis and decrease lipolysis when it is consumed at a level beyond what is utilized for maintenance and growth [17].
Although randomized clinical trials supporting such observations are lacking in older children, this suggests a potential upper limit in dietary protein utilization into lean tissue in younger children, although it is yet to be determined whether this phenomenon persists in later childhood. Therefore, it may be that children require distinct nutritional strategies enabling an adequate daily dietary protein intake which may in part be driven by daily physical activity levels as well as the age of the child [8].
Protein at Breakfast
It has long been acknowledged that the pattern and not just the absolute amount of dietary protein can influence nitrogen retention in both adults and children [18, 19]. According to the latest NHANES (National Health and Nutrition Examination Survey) data, the dietary intake patterns for protein and energy of children in the USA are skewed towards the evening meal occasion, with the breakfast occasion reporting the least intake on any given day while the evening occasion reporting up to ∼200% of the recommended daily intake for protein [20]. Studies in adults have shown that net protein balance is negative following an overnight fast with protein ingestion in the morning stimulating protein synthesis and inducing a positive net protein balance in lean tissues (including muscles) [21–23]. Moreover, the overnight fasted protein losses are offset by fed-state gains [24, 25], which demonstrates the diurnal variations observed in nitrogen and protein metabolism in weight-stable adults.
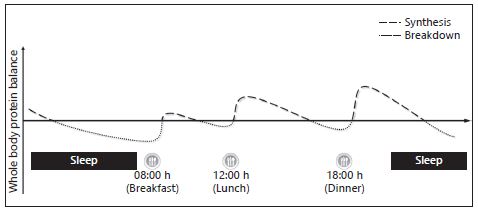
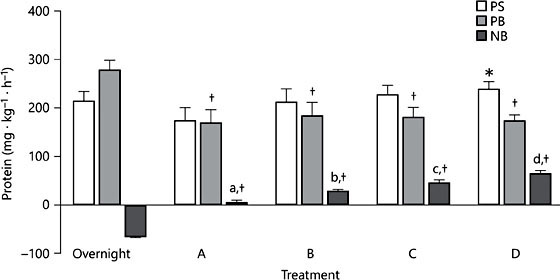
However, in the context of healthy children who undergo accelerated growth compared to adults, the breakfast occasion may actually be a key moment for protein ingestion in terms of promoting a physiological environment to sustain these growth requirements. Indeed, we recently reported that following a 10-h sleep, children were in a net negative state of whole body protein balance with whole body breakdown being greater than that of the rate of whole body protein synthesis [26] (Fig. 1). Furthermore, the ingestion of carbohydrates in the absence of protein at breakfast was enough to attenuate the observed overnight losses. However, the addition of protein at breakfast promoted a positive net whole body protein balance by stimulating increases in protein synthesis (Fig. 2). Therefore, the consumption of ∼7 g of milk proteins at breakfast in combination with carbohydrates may be an adequate nutritional strategy to promote lean tissue accrual.
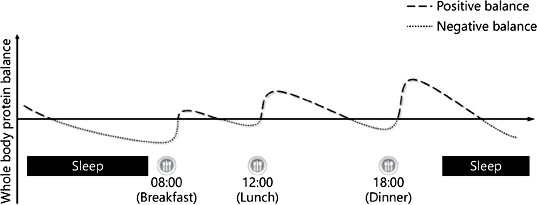
Fig. 1. Hypothetical diagram highlighting diurnal variations in whole body protein balance as a function of meal timing and quantity representative of Western eating behaviors.
Fig. 2. Relative whole-body protein turnover during the 9-h period after test beverage (breakfast) ingestion calculated using the [15N]-ammonia end-product method ex- pressed relative to body mass in children who consumed 0 g protein at breakfast (Group A, n = 16), 7 g protein at breakfast (Group B, n = 10), 14 g protein at breakfast (Group C, n = 14), or 21 g protein at breakfast (Group D, n = 15). Values are presented as means ± SEMs. Labeled means without a common letter differ, p < 0.05. * Different from Group A, p < 0.001; † different from corresponding overnight data, p < 0.01. NB, net balance; PB, protein breakdown; PS, protein synthesis [26]. Published with permission.
Dietary Protein Distribution in Children
In recent times, a great deal of current research has started focusing on the interaction between the circadian timing system, metabolic physiology, and nutritional science. These circadian oscillations are driven by the existence of under- lying intrinsic biological clocks with near 24-h periods. Understanding how such systems interact with the nutrient uptake and utilization at the metabolic level is currently being researched as a means to optimize metabolic functioning in response to nutrition. For example, it has long been reported that the pattern and not just the absolute amount of dietary protein can influence nitrogen retention in both adults and children [18, 19], as well as leucine balance in adults [27]. Recently, emphasis has been placed on the breakfast eating occasion with particular interest on the role of dietary protein intake at breakfast to enhance protein balance, which is negative following an overnight fast [28, 29]. Recent re- search in adults has demonstrated that an even distribution of dietary protein in adults (90 g of protein evenly throughout the day; 3 × 30 g) resulted in greater muscle fraction synthetic rates than consuming the same amount of protein (90 g) in a skewed fashion (10, 15, and 65 g at breakfast, lunch, and dinner, respectively) [30]. On the one hand, this may indicate the need to consume great- er amounts of protein at breakfast. On the other hand, this may highlight the importance of an overall threshold of protein intake that needs to be reached at each meal. Nonetheless, it seems that collectively current research suggests that meal protein intakes and distribution may help to optimize protein metabolism and net protein balance in adults [31].
However, to date, there is little research on the impact of discrete meal protein intake and daily distribution on protein metabolism and whole body net balance in healthy active children. In a recent 24-h controlled clinical trial, a skewed pattern of dietary protein intake seemed to promote greater gains in whole body protein balance compared to a more balanced daily protein intake in children consuming a high-protein diet [26]. It may be that the habitual daily dietary protein intake above the current recommendations for children could potentially mask any impact of the dietary pattern on whole body protein balance. For instance, it has previously been reported that greater intakes of dietary protein (2 g/kg · per day) result in reduced efficiency of postprandial nitrogen retention compared with normal protein intakes (1 g/kg · per day) [32].
In light of the previously reported associations and clinical trials linking in- creased protein intake with increased adiposity in children, further work should explore the longer-term implications of protein distribution on body composition. This in turn may elicit a viable way to provide the protein needs required for healthy physical growth in children while at the same time limiting the current excessive intake. Furthermore, better targeting the key moments in the life of a healthy physically active child, such as breakfast and the time following exercise/play, could likely provide an increased efficiency of dietary protein utilization while minimizing any detrimental effects of increased dietary protein intake in children.
Conclusion
In terms of nutrient availability, current research supports the concept of nutrient intake timing. Specifically, similar to adults, a typically observed overnight fast in children (i.e., a ∼10-h overnight fast) has recently been shown to result in a physiological state of increased catabolism as measured by increased rates of whole body protein breakdown. It is, therefore, important that specific amounts of protein be consumed at breakfast in order to attenuate such losses in whole body protein providing an environment that supports healthy physical growth and development. Specifically, consuming as little as 7 g of milk protein as part of a carbohydrate-containing breakfast seems to be sufficient to promote a positive net protein balance which persists up to 9 h following breakfast consumption when habitual diet and activity are controlled. Future research utilizing long-term studies are ultimately required to validate whether or not the benefits observed in the short term are translated to long-term benefits in terms of promoting increased lean tissue mass and, therefore, favorable body composition in healthy active children.
References
-
1 Almoosawi S, Vingeliene S, Karagounis LG, Pot GK: Chrono-nutrition: a review of cur- rent evidence from observational studies on global trends in time-of-day of energy intake and its association with obesity. Proc Nutr Soc 2016;75:487–500.
-
2 Nicklas TA, O’Neil C, Myers L: The importance of breakfast consumption to nutrition of children, adolescents, and young adults. Nutr Today 2004;39:30–39.
-
3 Betts JA, Richardson JD, Chowdhury EA, et al: The causal role of breakfast in energy balance and health: a randomized controlled trial in lean adults. Am J Clin Nutr 2014;100:539–547.
-
4 Boirie Y, Dangin M, Gachon P, et al: Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc Natl Acad Sci USA 1997;94:14930–14935.
-
5 Hoppe C, Molgaard C, Dalum C, et al: Differential effects of casein versus whey on fasting plasma levels of insulin, IGF-1 and IGF-1/ IGFBP-3: results from a randomized 7-day supplementation study in prepubertal boys. Eur J Clin Nutr 2009;63:1076–1083.
-
6 Rennie MJ, Edwards RH, Halliday D, et al: Muscle protein synthesis measured by stable isotope techniques in man: the effects of feeding and fasting. Clin Sci (Lond) 1982;63:519– 523.
-
7 Greenhaff PL, Karagounis LG, Peirce N, et al: Disassociation between the effects of amino acids and insulin on signaling, ubiquitin ligases, and protein turnover in human muscle. Am J Physiol Endocrinol Metab 2008; 295:E595–E604.
-
8 Rodriguez NR: Optimal quantity and composition of protein for growing children. J Am Coll Nutr 2005;24:150s–154s.
-
9 World Health Organization, Food and Agriculture Organization of the United Nations, United Nations University: Protein and Amino Acid Requirements in Human Nutrition. Report of a Joint FAO/WHO/UNU Expert Consultation. World Health Organ Tech Rep Ser 935. Geneva, WHO, 2007.
-
10 Tanner JM, Whitehouse RH: Clinical longitudinal standards for height, weight, height velocity, weight velocity, and stages of puberty. Arch Dis Child 1976;51:170–179.
-
11 Volterman KA, Moore DR, Breithaupt P, et al: Post exercise dietary protein ingestion in- creases whole-body leucine balance in a dose-dependent manner in healthy children.J Nutr 2017;147:807–815.
-
12 Volterman KA, Moore DR, Breithaupt P, et al: Timing and pattern of post exercise protein ingestion affects whole-body protein balance in healthy children: a randomized trial. Appl Physiol Nutr Metab 2017;42:1142–1148.
-
13 Gruszfeld D, Weber M, Gradowska K, et al: Association of early protein intake and preperitoneal fat at five years of age: follow-up of a randomized clinical trial. Nutr Metab Car- diovascDis 2016;26:824–832.
-
14 Eloranta AM, Lindi V, Schwab U, et al: Dietary factors associated with overweight and body adiposity in Finnish children aged 6–8 years: the PANIC Study. Int J Obes (Lond) 2012;36:950–955.
-
15 Voortman T, Braun KV, Kiefte-de Jong JC, et al: Protein intake in early childhood and body composition at the age of 6 years: the Generation R Study. Int J Obes (Lond) 2016;40: 1018–1025.
-
16 Jen V, Karagounis LG, Jaddoe VWV, et al: Dietary protein intake in school-age children and detailed measures of body composition: the Generation R Study. Int J Obes (Lond) 2018, Epub ahead of print.
-
17 Koletzko B, Broekaert I, Demmelmair H, et al: Protein intake in the first year of life: a risk factor for later obesity? The E.U. childhood obesity project. Adv Exp Med Biol 2005;569: 69–79.
-
18 Gattas V, Barrera GA, Riumallo JS, Uauy R: Protein-energy requirements of prepubertal school-age boys determined by using the nitrogen-balance response to a mixed-protein diet. Am J Clin Nutr 1990;52:1037–1042.
-
19 Leverton RM, Gram MR: Nitrogen excretion of women related to the distribution of animal protein in daily meals. J Nutr 1949;39:57–65.
-
20 Mathias KC, Almoosawi S, Karagounis LG: Protein and energy intakes are skewed to- ward the evening among children and adolescents in the United States: NHANES 2013– 2014. J Nutr 2017;147:1160–1166.
-
21 Volpi E, Mittendorfer B, Wolf SE, Wolfe RR: Oral amino acids stimulate muscle protein anabolism in the elderly despite higher first-pass splanchnic extraction. Am J Physiol 1999;277:E513–E520.
-
22 Volpi E, Ferrando AA, Yeckel CW, et al: Exogenous amino acids stimulate net muscle protein synthesis in the elderly. J Clin Invest 1998;101:2000–2007.
-
23 Rennie MJ, Edwards RHT, Halliday D, et al: Muscle protein synthesis measured by stable isotope techniques in man: the effects of feeding and fasting. Clin Sci 1982;63:519–523.
-
24 Pacy PJ, Price GM, Halliday D, et al: Nitrogen homoeostasis in man: the diurnal responses of protein synthesis and degradation and amino acid oxidation to diets with increasing protein intakes. Clin Sci 1994;86:103–118.
-
25 Price GM, Halliday D, Pacy PJ, et al: Nitrogen homoeostasis in man: influence of protein intake on the amplitude of diurnal cycling of body nitrogen. Clin Sci 1994;86: 91–102.
-
26 Karagounis LG, Volterman KA, Breuillé D, et al: Protein intake at breakfast promotes a positive whole-body protein balance in a dose-response manner in healthy children: a randomized trial. J Nutr 2018;148:729– 737.
-
27 el-Khoury AE, Sánchez M, Fukagawa NK, et al: The 24-h kinetics of leucine oxidation in healthy adults receiving a generous leucine intake via three discrete meals. Am J Clin Nutr 1995;62:579–590.
-
28 Leidy HJ, Gwin JA, Roenfeldt CA, et al: Evaluating the intervention-based evidence surrounding the causal role of breakfast on markers of weight management, with specific focus on breakfast composition and size. Adv Nutr 2016;7:563S–575S.
-
29 Leidy HJ, Hoertel HA, Douglas SM, et al: A high-protein breakfast prevents body fat gain, through reductions in daily intake and hunger, in “breakfast skipping” adolescents. Obesity (Silver Spring) 2015;23:1761–1764.
-
30 Mamerow MM, Mettler JA, English KL, et al: Dietary protein distribution positively influences 24-h muscle protein synthesis in healthy adults. J Nutr 2014;144:876– 880.
-
31 Layman DK, Anthony TG, Rasmussen BB, et al: Defining meal requirements for protein to optimize metabolic roles of amino acids. Am J Clin Nutr 2015;101:1330S–1338S.
-
32 Morens C, Bos C, Pueyo ME, et al: Increasing habitual protein intake accentuates differences in postprandial dietary nitrogen utilization between protein sources in humans. J Nutr 2003;133:2733–2740.