Human Milk Proteins: Composition and Physiological Significance
Abstract
Human milk (HM) contains hundreds of proteins with very diverse functions that likely contribute to the short- and long-term beneficial effects of breastfeeding. These functions include serving as a source of amino acids, improving the bioavailability of micronutrients, including vitamins, minerals, and trace elements, providing immunologic defense, stimulating intestinal growth and maturation, shaping the microbiome, and enhancing learning and memory. Human milk proteins can be broadly classified into 3 categories: caseins, whey proteins, and mucins, which are present in the milk fat globule membrane. HM is whey predominant; however, the whey/casein ratio of HM changes from 90/10 in colostrum to 60/40 in mature HM. The whey proteins present in significant quantities in the whey fraction are α-lactalbumin, lactoferrin, IgA, osteopontin, and lysozyme. Additionally, bioactive peptides are formed during digestion of casein and whey, and glycans from glycoproteins are bifidogenic, adding further complexity to the functional properties of HM proteins. Recent advances in dairy technology have enabled isolation of bioactive milk proteins from bovine milk in sufficient quantities for clinical studies and, in some cases, addition to commercially available infant formula. Herein, the current evidence on HM protein composition and bioactivity of HM proteins is reviewed.
Introduction
Human milk (HM) provides nutrients in readily bioavailable forms that ensure optimal infant growth and development [1]. HM also contains a variety of bio-active proteins, lipids, and oligosaccharides that serve nonnutritional roles [2]. Over the past 30 years, infant formula composition has evolved to more closely mimic that of HM [3]. However, despite these attempts, disparities in growth [4], neurodevelopment [5], microbiome composition [6], immune function [7], and infectious disease incidence [8] persist between breast- and formula-fed infants. It is thought that the bioactive components in HM are in part responsible for these developmental differences. Herein, HM proteins, which provide indispensable amino acids for growth as well as bioactive proteins and peptides that serve nonnutritional roles in the developing neonate, will be reviewed [9].
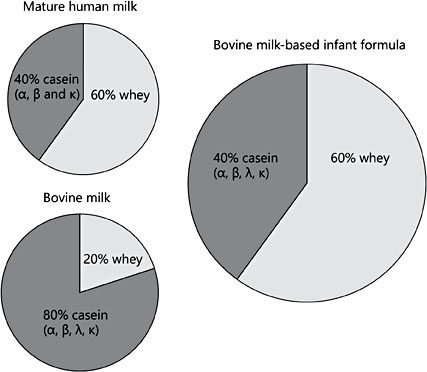
Fig. 1. Comparison of the whey/casein ratios of mature human milk, bovine milk, and standard commercial infant formulas.
Human Milk Protein Composition
The estimated 400 HM proteins [10, 11] are classified into 3 categories: caseins, whey proteins and mucins, the latter of which are present in the milk fat globule membrane (MFGM) [12]. Bovine milk is casein predominant, whereas HM is whey predominant. The HM whey/casein ratio changes over the course of lactation, declining from 90/10 in colostrum (days [d] 0–5) to 65/35 in transitional milk (d6–15), then 60/40 by 1 month postpartum and throughout the first year of lactation [12]. Bovine milk contains α-, β-, γ-, and κ-caseins, whereas HM contains β- and κ-casein, with lower concentrations of α-casein (Fig. 1). The whey/casein ratio in formula is similar to mature HM (60/40), but formula contains all bovine milk caseins (Fig. 1). The concentrations of total and β- and κ-casein slightly rise between early and transitional milk, before declining and remaining relatively stable in mature milk. In contrast, the concentration of α-casein is constant throughout lactation (Table 1) [13].
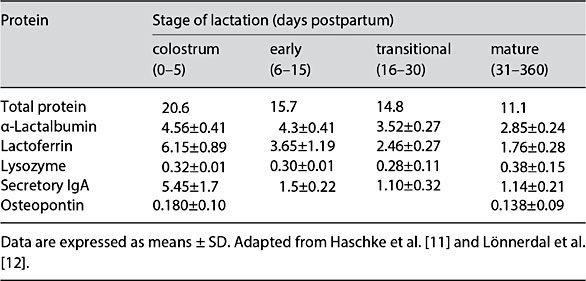
The HM whey proteins present in highest concentration are α-lactalbumin, lactoferrin (LF), secretory IgA, osteopontin (OPN), and lysozyme (Table 2) [9, 11, 14]. In general, concentrations of these proteins sharply decrease from colostrum to mature HM, with the exception of lysozyme, which remains relatively steady [9]. Proteomic analysis of HM whey identified 115 unique proteins, of which 35% were related to immune response [15]. Other key functions were cell communication (17% of proteins), metabolism/energy production (16%), and general transport (12%) [15]. Proteomic analysis of the MFGM identified 191 proteins, with functions enriched in metabolism/ energy production (21%), cell communication (19%), and general transport (16%), and to a lesser degree immune response (20%) compared to whey proteins [15, 16]. This paper identified many new MFGM proteins and pro- vided insight into potential significance of MFGM for infant nutrition [16]. The predominant whey protein in bovine milk is β-lactoglobulin, which is not present in HM. Bovine milk also contains less α-lactalbumin, LF, and OPN than HM, with LF and OPN concentrations being 5 and 13% of HM concentrations, respectively [3, 17, 18]. Advances in dairy technology have enabled the isolation of bioactive milk proteins from bovine milk in sufficient quantities for clinical studies and, in some cases, addition to infant formulas [3, 19].
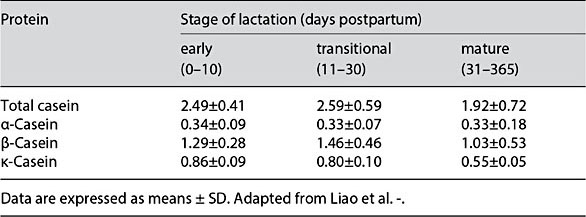
Table 1. Concentrations (g/L) of total casein and casein subunits in human milk over the first year of lactation
Table 2. Concentrations (g/L) of total protein and major whey proteins in human milk over the first year of lactation
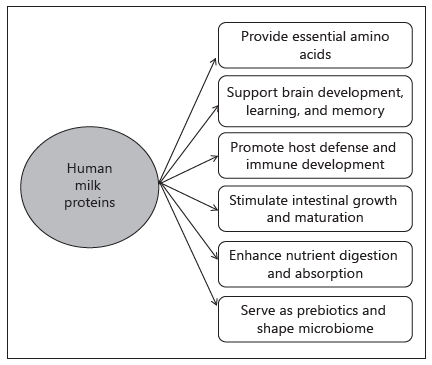
Fig. 2. Biological functions of human milk proteins.
Biological Activities of HM Proteins
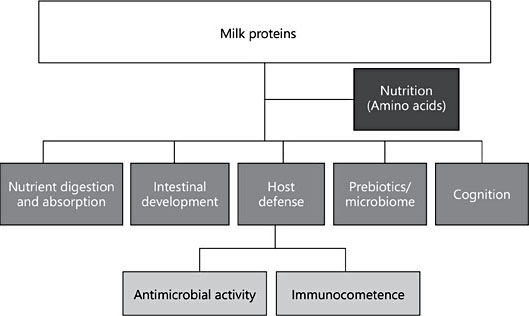
HM proteins exert a range of functions including: serving as a source of amino acids; improving micronutrient bioavailability; stimulating intestinal growth and maturation; supporting immunologic defense; shaping the microbiome; and enhancing learning and memory (Fig. 2). Some HM proteins exert activities across several categories (Table 3). For example, LF has been implicated as a nutrient transporter, in host defense, and for promoting intestinal, cognitive, and immune functions [18, 20–22] (Table 3). Additionally, bioactive peptides are formed during digestion of casein and whey proteins [12], and glycans released from HM glycoproteins by microbial glycosidases are bifidogenic [23], adding further complexity to the functional properties of HM proteins. Due to their multifunctional roles, 3 bioactive proteins, LF, OPN, and MFGM, have been isolated from bovine milk and tested for bioactivity in preclinical studies and human clinical trials [18].
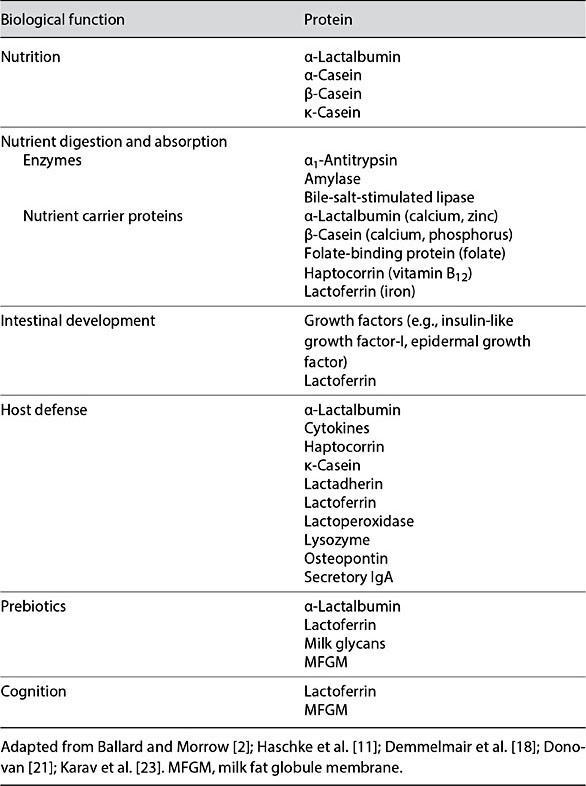
Table 3. Human milk proteins associated with biological function
Bioactivities of Lactoferrin
LF is a nonheme iron binding protein that is one of the most widely studied HM proteins [20]. Randomized controlled clinical trials (RCTs) have sup- ported antimicrobial and immunomodulatory activities of LF. For example, feeding 1.0 g/day of bovine LF for 9 months reduced Giardia lamblia colonization and increased growth of 12- to 36-month-old infants in Peru [24]. The addition of recombinant human LF (1.0 g/L) and lysozyme (0.2 g/L) to an oral rehydration solution reduced the duration of diarrhea in 5- to 35-month- old Peruvian infants hospitalized for diarrhea [25]. A Cochrane review concluded that “evidence of moderate to low quality suggests that oral LF pro- phylaxis with or without probiotics decreases late-onset sepsis and necrotizing enterocolitis stage II or greater in preterm infants without adverse effects” [26]. Thus, dietary LF has efficacy in both preventing and treating infectious diseases.
Orally administered LF exerts antibacterial and antiviral activities in the intestine through direct effects on pathogens and by affecting gastrointestinal and immune function [20, 21]. The latter functions are mediated by LF being taken up by cells via receptor-mediated pathways and affecting gene transcription [27]. In piglets, dietary bovine LF (1.0 or 3.6 g/L) increased intestinal cell proliferation, crypt depth, and β-catenin expression threefold [reviewed in 21]. Also, in piglets, dietary bovine LF modulated both systemic and intestinal immune development by stimulating a balanced T-helper-1/T-helper-2 cytokine response. Further, immune cells from piglets fed LF secreted more anti-inflammatory cytokines in an unstimulated state, while being primed for more a robust proinflammatory response when presented with a bacterial trigger ex vivo [re- viewed in 21].
In terms of cognitive development, piglets fed bovine LF (0.6 g/L) from d3–d38 postpartum exhibited improved learning and memory in an 8-arm radial maze test compared with piglets fed an unsupplemented formula [22]. Moreover, ingested LF was associated with differential expression of 10 genes involved in the brain-derived neurotrophin factor (BDNF) signaling pathway in the hippocampus and upregulated the expression of polysialic acid, a marker of neuroplasticity, cell migration, and differentiation of progenitor cells, and the growth and targeting of axons, and increased phosphorylation of the cyclic adenosine monophosphate response element-binding protein, CREB, a downstream target of the BDNF signaling pathway, and an important protein in neurodevelopment and cognition [22]. Taken together, feeding LF at HM concentrations was bioactive with no reported adverse effects. LF is currently being supplemented to some commercial infant formulas; however, there are likely untapped potentials for LF to address some of the current immune, health, and cognitive differences be- tween breast- and formula-fed infants; however, additional clinical trials are needed.
Bioactivities of Osteopontin
OPN is an acidic, glycosylated, and highly phosphorylated protein. It interacts with cell surface integrins and the CD44 receptor to influence biomineralization, tissue remodeling, and immune regulation [14, 18]. Feeding a formula with bovine OPN at the mean HM concentration to rhesus monkeys affected the expression of ∼2,000 intestinal genes and shifted the overall pattern of expression to be more similar to breastfed monkeys [28]. OPN influenced genes related to cell proliferation, migration, communication, and survival, and genes in pathways downstream from integrin and CD44 receptors [28]. In a recent RCT, formulas with either 65 or 130 mg bovine OPN/L was well tolerated and supported nor- mal growth. However, infants consuming bovine OPN had a lower incidence of fever than standard formula and similar to breastfed controls [29]. Both OPN- supplemented infants had lower levels of TNF-α, higher levels of interleukin-2 and higher proportions of CD3+CD45+ T cells compared to the standard formula group [29, 30]. These studies indicate that oral OPN beneficially affects neonatal intestinal and immune development.
Bioactivities of MFGM
MFGM is the triple membrane system that encapsulates milk fat [31]. It contains cholesterol, glycerol-phospholipids, sphingolipids, and proteins, including mucin-1, butyrophilin, CD36, adipophilin, and lactadherin, which contribute to the antiviral and antibacterial activities of MGFM [31]. Indeed, an observational study of infants during the first 6 months of life found that rotavirus infection was negatively associated with the amount of HM lactadherin consumed, while intake of mucin and secretory IgA with milk was unrelated [32]. A recent RCT tested the safety and efficacy of MFGM in term infants randomized before the age of 2 months to a formula supplemented with a protein-rich MFGM prepara- tion (4% of total protein) or a standard formula [reviewed in 31]. Formulas were fed until 6 months of age, the infants were followed until 12 months, and they were compared with a breastfed reference group. MFGM reduced diarrhea, oti- tis media, fever, and pyretic use [reviewed in 31]. Interestingly, Moraxella catarrhalis, a microbe commonly found in the middle ear in otitis media, was less abundant in the saliva of infants fed formula with MFGM, providing a potential mechanism of action [32]. In addition, the MFGM-supplemented group (105.8 ± 9.2) had significantly higher mean (± SD) scores in the cognitive do- main of the Bayley Scales of Infant and Toddler Development compared to the standard formula group (101.8 ± 8.0) [reviewed in 31]. Notably, the MFGM- supplemented formula-fed infants achieved cognitive scores not different than the breastfed infants (106.4 ± 9.5). Taken together, these studies establish the multifunctional actions of a single ingredient (OPN) in reducing the differences in cognitive and immune outcomes between breast- and formula-fed infants.
Future Directions
HM or formula provides the sole source nutrition of the first 6 months of life, which is a critical period of infant growth and development [1, 8]. Recent RCTs have shown that the bioactive proteins, LF, OPN, and MFGM, isolated from bovine milk, have beneficial effects on immune and cognitive outcomes in healthy term infants in the short term [31]. Future studies should investigate combinations of bioactive components (these HM proteins and other components, such as HM oligosaccharides or lipids). In addition, potential effects on longer-term programming of the immune system and cognitive functions and health outcomes must be investigated by following these infants in these study cohorts into later childhood.
References
- 1 American Academy of Pediatrics, Section on Breastfeeding. Breastfeeding and the use of human milk. Pediatrics 2012;129:e827–e841.
- 2 Ballard O, Morrow AL: Human milk composition: nutrients and bioactive factors. Pediatr Clin North Am 2013;60:49–74.
- 3 Hernell O: Human milk vs cow’s milk and the evolution of infant formulas. Nestle Nutr Workshop Ser Pediatr Program 2011;67:17– 28.
- 4 Bell KA, Wagner CL, Feldman HA, et al: Associations of infant feeding with trajectories of body composition and growth. Am J Clin Nutr 2017;106:491–498.
- 5 Bar S, Milanaik R, Adesman A: Long-term neurodevelopmental benefits of breastfeeding. Curr Opin Pediatr 2016;28:559–566.
- 6 Davis EC, Wang M, Donovan SM: The role of early life nutrition in the establishment of gut microbial composition and function. Gut Microbes 2017;8:143–171.
- 7 Munblit D, Treneva M, Peroni DG, et al: Immune components in human milk are associated with early infant immunological health outcomes: a prospective three-country analysis. Nutrients 2017;9:E532.
- 8 Victora CG, Bahl R, Barros AJ, et al: Breast- feeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet 2016; 387:475–490.
- 9 Lönnerdal B: Bioactive proteins in human milk: health, nutrition, and implications for infant formulas. J Pediatr 2016; 173(suppl):S4–S9.
- 10 Andreas NJ, Kampmann B, Mehring Le-Do- are K: Human breast milk: a review on its composition and bioactivity. Early Hum Dev 2015;91:629–635.
- 11 Haschke F, Haiden N, Thakkar SK: Breast- milk: Nutritive and bioactive proteins. Ann Nutr Metab 2016;69(suppl 2):17–26.
- 12 Lönnerdal B, Erdmann P, Thakkar SK, et al: Longitudinal evolution of true protein, amino acids and bioactive proteins in breast milk: a developmental perspective. J Nutr Biochem 2017;41:1–11.
- 13 Liao Y, Weber D, Xu W, et al: Absolute quantification of human milk caseins and the whey/casein ratio during the first year of lactation. J Proteome Res 2017;16:4113–4121.
- 14 Schack L, Lange A, Kelsen J, et al: Considerable variation in the concentration of osteopontin in human milk, bovine milk, and infant formulas. J Dairy Sci 2009;92:5378–5385.
- 15 Liao Y, Alvarado R, Phinney B, et al: Proteomic characterization of human milk whey proteins during a twelve-month lactation period. J Proteome Res 2011;10:1746–1754.
- 16 Liao Y, Alvarado R, Phinney B, Lönnerdal B: Proteomic characterization of human milk fat globule membrane proteins during a 12 month lactation period. J Proteome Res 2011; 10:3530–3541
- 17 Chatterton DEW, Nguyen DN, Bering SB, et al: Anti-inflammatory mechanisms of bioactive milk proteins in the intestine of newborns. Int J Biochem Cell B 2013;45:1730– 1747.
- 18 Demmelmair H, Prell C, Timby N, et al: Benefits of lactoferrin, osteopontin and milk fat globule membranes for infants. Nutrients 2017;9:E817.
- 19 Lönnerdal B: Infant formula and infant nutrition: bioactive proteins of human milk and implications for composition of infant formulas. Am J Clin Nutr 2014;99:712S–717S.
- 20 Lönnerdal B: Nutritional roles of lactoferrin. Curr Opin Clin Nutr Metab Care 2009;12: 293–297.
- 21 Donovan SM: The role of lactoferrin in gastrointestinal and immune development and function: a preclinical perspective. J Pediatr 2016;173S:S16–S28.
- 22 Chen Y, Zheng Z, Zhu X, et al: Lactoferrin promotes early neurodevelopment and cognition in postnatal piglets by upregulating the BDNF signaling pathway and polysialylation. Mol Neurobiol 2015;52:256–269.
- 23 Karav S, Le Parc A, Leite Nobrega de Moura Bell JM, et al: Oligosaccharides released from milk glycoproteins are selective growth substrates for infant-associated bifidobacteria. Appl Environ Microbiol 2016;82:3622–3630.
- 24 Ochoa TJ, Chea-Woo E, Campos M, et al: Impact of lactoferrin supplementation on growth and prevalence of Giardia colonization in children. Clin Infect Dis 2008;46: 1881–1883.
- 25 Zavaleta N, Figueroa D, Rivera J, et al: Efficacy of rice-based oral rehydration solution containing recombinant human lactoferrin and lysozyme in Peruvian children with acute diarrhea. J Pediatr Gastroenterol Nutr 2007; 44:258–264.
- 26 Pammi M, Abrams SA: Oral lactoferrin for the prevention of sepsis and necrotizing en- terocolitis in preterm infants. Cochrane Database Syst Rev 2015;2:CD007137.
- 27 Liao Y, Jiang R, Lönnerdal B: Biochemical and molecular impacts of lactoferrin on small intestinal growth and development during early life. Biochem Cell Biol 2012;90:476–484.
- 28 Donovan SM, Monaco MH, Drnevich J, et al: Bovine osteopontin modifies the intestinal transcriptome of formula-fed infant rhesus monkeys to be more similar to those that were breastfed. J Nutr 2014;144:1910–1919.
- 29 Lönnerdal B, Kvistgaard AS, Jacobsen LN, et al: Growth, nutritional status and cytokine response of breast-fed infants and infants fed formula with added bovine osteopontin in the first six months of life. J Pediatr Gastroenterol Nutr 2016;62:650–657.
- 30 West CE, Kvistgaard AS, Peerson JM, et al: Effects of osteopontin-enriched formula on lymphocyte subsets in the first 6 months of life: a randomized controlled trial. Pediatr Res 2017;82:63–71.
- 31 Timby N, Domellöf M, Lönnerdal B, et al: Supplementation of infant formula with bovine milk fat globule membranes. Adv Nutr 2017;8:351–355.
- 32 Newburg DS, Peterson JA, Ruiz-Palacios GM, et al: Role of human-milk lactadherin in protection against symptomatic rotavirus infection.Lancet 1998;351:1160–1164.
- 33 Timby N, Domellöf M, Holgerson PL, et al: Oral microbiota in infants fed a formula sup- plemented with bovine milk fat globule membranes – a randomized controlled trial. PLoS One 2017;12:e0169831.