Human Milk Oligosaccharides: Next Generation Functions and Questions
Abstract
Human milk oligosaccharides (HMOs) are the third most abundant component of human milk. So far, more than 150 different and structurally distinct HMOs have been identified. HMO composition varies substantially between women, but remains fairly constant over the course of lactation in the same woman. Which maternal genetic and environmental factors drive the interindividual variations in HMO composition remains poorly understood, and it is currently unknown whether or not a woman’s characteristic HMO composition has evolved to match her own infant’s specific needs. A combination of preclinical, cohort and clinical studies is required to fully assess the many effects, functions and potential claims associated with HMOs.
In some cases, individual HMOs exert a certain effect and, while there might be some redundancy, the effects are often highly structure-specific. In other cases, a combination of different HMOs in specific ratios to each other is required to be effective, and future research needs to assess whether or not the administration of individual HMOs alone may be counterproductive and potentially harmful to the infant’s short- and long-term health. Overall, the personalized complexity of HMOs cannot be mimic in artificial infant formula and provides yet another powerful reason to protect, promote and support breastfeeding.
What are human milk oligosaccharides (HMOs)?
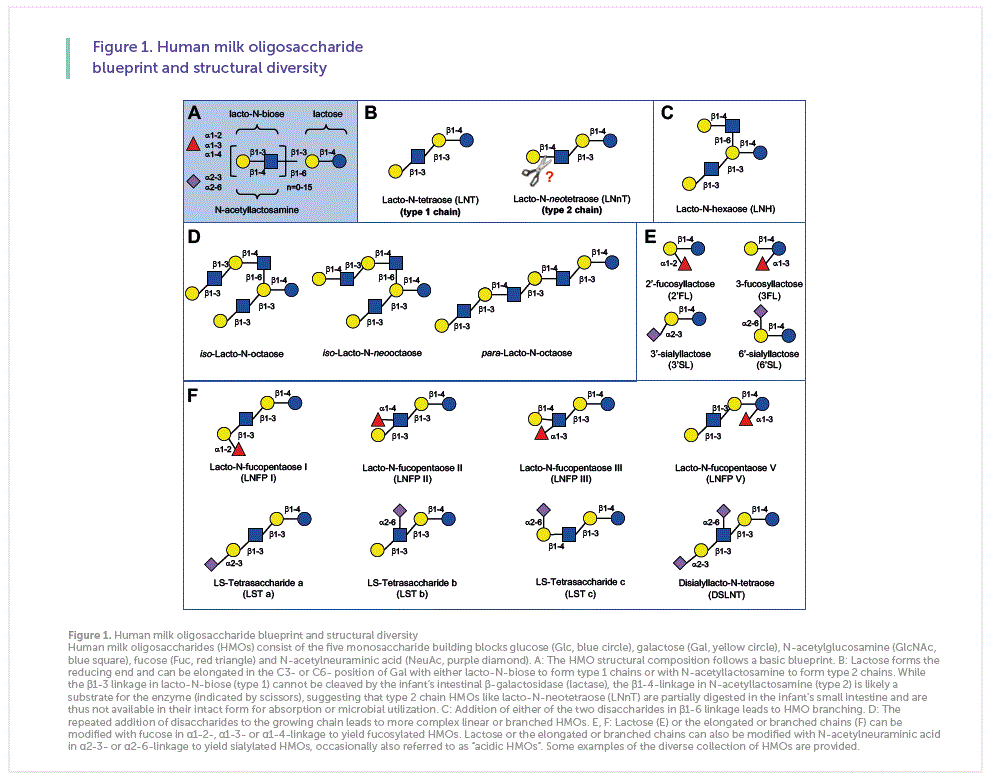
One Liter of mature human milk contains 5-15 g of complex carbohydrates that are collective called human milk oligosaccharides (HMOs). Their high concentration makes HMOs the third most abundant solid component of mature milk, only exceeded by the concentration of lactose and total lipids and often exceeding the concentration of total proteins [1]. In comparison, the concentration of oligosaccharides in bovine milk is 100- to 1,000-fold lower and many of the bovine milk oligosaccharides are structurally distinct from and less complex than HMOs. While more than 150 different HMOs have been identified so far, their composition follows a basic blueprint that connects the five building blocks glucose (Glc), galactose (Gal), N-acetylglucosamine (GlcNAc), fucose (Fuc), and N-acetylneuraminic acid (Neu5Ac) in specific linkages (Figure 1A) [2]. All HMOs carry lactose (Galβ1-4Glc) at the reducing end. Lactose can be elongated in the C3 position of Gal with either lacto-N-biose (Galβ1-3GlcNAc, type 1 chain) or N-acetyllactosamine (Galβ1-4GlcNAc, type 2 chain) (Figure 1B). This ostensibly minor difference in the linkage between Gal and GlcNAc might be important for HMO digestion and bioavailability. While the β1-3 linkage in lacto-N-biose (type 1) cannot be cleaved by the infant’s intestinal β-galactosidase (lactase), the β1-4-linkage in
N-acetyllactosamine (type 2) is likely a substrate for the enzyme, suggesting that type 2 chain HMOs like lacto-N- neotetraose (LNnT) are partially digested in the infant’s proximal small intestine, do not reach the infant’s distal small intestine and colon intact, and are therefore not available for absorption or microbial utilization. In contrast, type 1 chain HMOs like lacto-N-tetraose (LNT) cannot be cleaved by lactase, resist degradation in the infant’s small intestine, reach the colon intact, and are available for absorption or microbial utilization. In addition to chain elongation at the C3 position of Gal, the disaccharides lacto-N-biose or N-acetyllactosamine can also be added at the C6 position of Gal, leading to chain branching (Figure 1C).
The repeated addition of disaccharides to the growing chain leads to more complex linear or branched HMOs (Figure 1D). Moreover, HMOs can be modified by the addition of Fuc in α1-2-, α1-3-, and/or α1-4-linkage and/or the addition of Neu5Ac in α2-3- and/or α2-6-linkage. Each Neu5Ac contains a carboxyl group and introduces a negative to an HMO. Fuc or Neu5Ac can be added to lactose to yield the small HMO trisaccharides 2’-fucosyllactose (2’FL), 3-fucosyllactose (3’FL), 3’-sialyllactose, or 6’-sialyllactose (Figure 1E). One or more Fuc and/or Neu5Ac can also be added to the growing linear or branched HMO chain to form complex HMOs with potentially multiple negative charges if more than one Neu5Ac are added (Figure 1F). Neu5Ac-containing HMOs are often referred to as “acidic” HMOs.
Although HMO composition follows a basic blueprint and more than 150 different HMOs have been identified so far, it is important to note that every woman synthesizes and secretes a distinct HMO composition profile that varies substantially between different women, but remains fairly constant over the course of lactation for the same woman.
What drives the variation in HMO composition?
Some of the variation in HMO composition can be explained by maternal genetics [1]. HMO fucosylation is mainly catalyzed by two different fucosyltransferases, enzymes that facilitate the addition of Fuc to the HMO backbone in different linkages [3,4]. Fucosyltransferase 2 (FUT2) adds Fuc to Gal in an α1-2-linkage [5]. Mutations in the Secretor gene, which encodes FUT2, lead to an inactivation of the enzyme, and, as a consequence, milk samples of so called nonsecretor women have very low concentrations of α1-2-fucosylated HMOs, e.g. 2’-FL or lacto-N-fucopentaose 1 (LNFP1). Fucosyltransferase 3 (FUT3) adds Fuc to Gal or GlcNAc in an α1-3- or α1-4-linkage, depending on whether the substrate is a type 1 or type 2 HMO chain [6]. Mutations in the Lewis gene, which encodes FUT3, lead to an inactivation of the enzyme, and, as a consequence, milk samples of so called Lewis-negative women have very low concentrations of α1-3/4-fucosylated HMOs.
The permutation of active/inactive FUT2 and FUT3 enzymes lead to four distinct HMO groups [4]: i. Lewis-positive secretors (FUT2 and FUT 3 active), ii. Lewis-positive nonsecretors (FUT2 inactive, but FUT3 active), iii. Lewis- negative secretors (FUT2 active, but FUT3 inactive), and iv. Lewis-negative nonsecretors (both FUT2 and FUT3 inactive). These four groups can be distinguished by the presence or near absence of specific fucosylated HMOs, which is almost an all-or-nothing phenomenon. For example, 2’FL is either present and one of the dominant HMOs in the milk of secretor women (active FUT2), or it is almost completely absent in the milk of nonsecretor women (inactive FUT2). In contrast, the variation of HMOs that do not depend on FUT2 or FUT3 activity is subtler. In fact, it remains poorly understood which enzymes other than FUT2 and FUT3 are involved in HMO biosynthesis in the human mammary gland epithelial cell.
In addition to maternal genetics and potentially epigenetics, maternal environmental factors may also affect HMO composition. Our recent work with mice has shown that milk oligosaccharide concentrations decrease when lactating dams are fed a high fat diet and increase when dams are exercising (unpublished data). How diet, exercise, and other lifestyle factors impact HMO composition in humans is currently under investigation. Maternal health may also affect HMO composition. Initial preliminary data suggest that obesity or chronic inflammatory diseases impact HMO composition (unpublished data). In addition, cross- sectional data from the Canadian Healthy Infant Longitudinal Development (CHILD) national birth cohort [7] indicate that parity increases overall HMO concentration, but maternal age, method of delivery, or infant gender showed no association with HMO composition (unpublished data).
What happens to HMOs after ingestion?
Once ingested, HMOs resist the low stomach pH as well as degradation through pancreatic and brush border enzymes in the small intestine [8,9], with the potential exception of type 2 chains in which the terminal β1-4-linked Gal may be cleaved off by the enzyme lactase. Approximately 1% of the ingested HMOs are absorbed and can be measured in the systemic circulation as well as in the urine [10-13], indicating that HMO effects extend to tissues and organs other than the intestine. Most HMOs reach the distal small intestine and colon intact where they are either metabolized by microbes or excreted with the feces.
What are potential HMO functions?
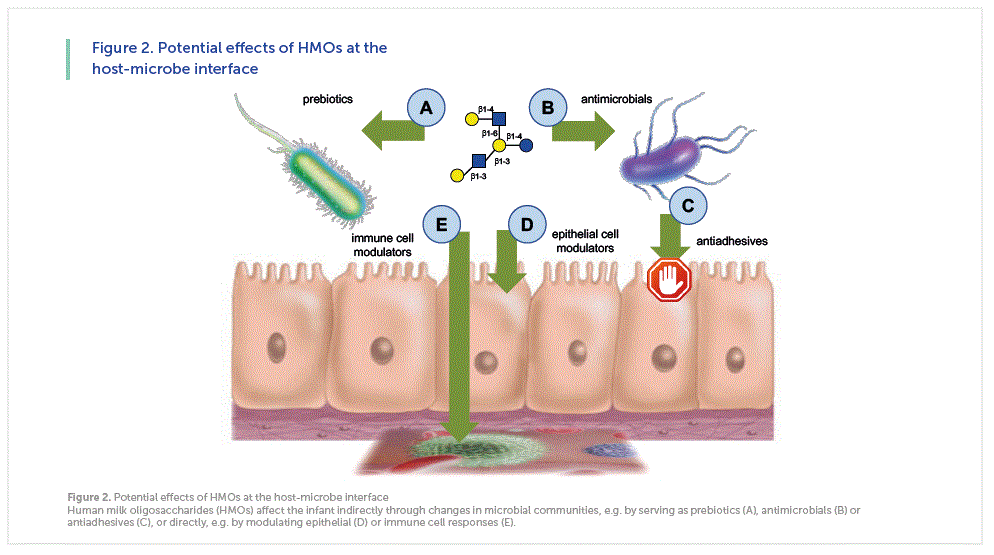
HMOs serve as metabolic substrates for specific and potentially health-promoting bacteria in the infant’s intestine [14-16], making them the first prebiotics that humans are exposed to in life – when the infant is breastfed (Figure 2A). However, recent data from ex vivo studies suggest that the prebiotic effects of different HMOs are not interchangeable and are indeed structure-specific (unpublished data). The composition of microbial communities isolated from infant fecal samples and cultured under anaerobic conditions changes over time depending on what specific HMOs are added to the culture. For example, the composition of a microbial community looks very different when exposed to either to a mixture of HMOs that were isolated from pooled human milk or to individual HMOs like 2’FL or LNT. Since the differential composition and activity of microbial communities has been linked to diseases like obesity, diabetes, inflammatory bowel disease or autism, exposing infants to individual HMOs in formula instead of a complex HMO mixture in human milk may increase disease risks. Long-term follow-up studies are required to rule out this potential risk and avoid potential harm to the infant’s short- and long-term health.
However, HMOs are more than just “food for bugs”. HMOs can have direct bacteriostatic or bacteriocidal antimicrobial effects (Figure 2B). For example, HMOs halt the growth of Streptococcus agalactiae (group B Streptococcus; GBS) [17], a leading cause of invasive bacterial infections in newborns, typically acquired vertically during childbirth secondary to maternal vaginal colonization. GBS transmission to the newborn is associated with risk of pneumonia, septicemia, and meningitis [18-20]. Multidimensional chromatography revealed that the bacteriostatic effect is structure dependent and that LNT is most effective. GBS transposon mutant library screening identified a GBS glycosyltransferase as the HMO target. Most intriguingly, the effects of HMOs synergize with common antibiotics like vancomycin and ciprofloxacin, with high relevance to the emerging antibiotic-resistance crisis [17].
HMOs also have antiadhesive effects (Figure 2C). Many pathogens need to attach to epithelial surfaces in order to proliferate and potentially invade host tissues. The attachment is often facilitated by pathogen surface molecules that bind to glycan structures on the epithelial cell surface, also known as the glycocalyx. HMOs and glycans on epithelial cell surfaces share many structural features, allowing HMOs to mimic epithelial surface glycans and serve as soluble decoy receptors. Instead of pathogens binding to epithelial surfaces and causing disease, they bind to the soluble HMO decoys and are washed out with attaching and without causing disease. Examples for antiadhesive effects of HMOs include bacterial pathogens like Campylobacter jejuni [21] or enteropathogenic E. coli (EPEC) [22] as well as protozoan parasites like Entamoeba histolytica [23].
HMOs may also have direct effects on epithelial cells, independent of, but indirectly affecting microbes (Figure 2D). For example, bladder epithelial cells that had been exposed to HMOs are significantly more resistant to uropathogenic E. coli invasion and cytotoxicity [24]. HMOs may also alter immune cell responses, either locally in the gut or systemically (Figure 2E).
In conclusion, there are indirect effects of HMOs on the infant that are mediated through changes in microbial communities, e.g. by serving as prebiotics, antimicrobials or antiadhesives, and there are direct effects on the infant, e.g. by modulating epithelial or immune cell responses. In addition, there may be multiple other mechanisms of HMO action that have not yet been discovered. Overall, the arsenal of HMO effects is likely relevant in a variety of health and disease contexts.
How do we interrogate the effects of HMOs?
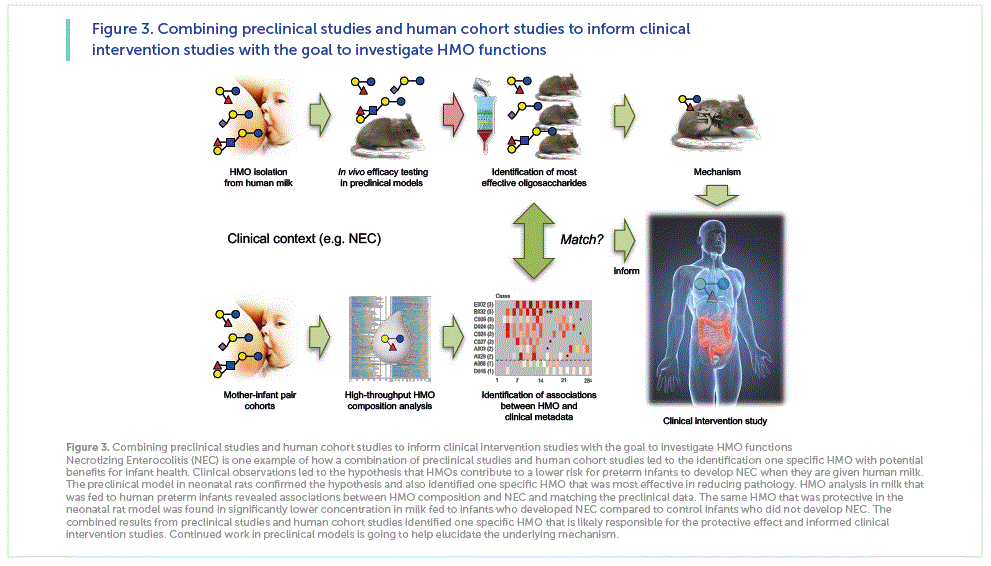
Accumulating data from tissue culture, animal or human cohort studies suggests that HMOs benefit the human milk-fed infant. However, results from many of these studies raise additional questions. How relevant are results generated from in vitro or animal models? How similar are cell lines in culture compared to tissues in the infant body? How comparable are animal models to human anatomy, physiology, and pathophysiology? How meaningful are associations between HMOs and infant outcome measures in human cohort studies? Are the observed associations true cause-and-effect relationships? No one single study alone will likely provide a comprehensive answer. Instead, a thoroughly designed interrogative strategy that combines preclinical studies in tissue culture and animal models with clinical cohort analyses and eventually clinical intervention studies will be required to fully assess the many effects, functions and potentials claims associated with HMOs. Our recent work on necrotizing enterocolitis (NEC) serves as one example of how the combination of preclinical studies and human cohort studies can inform clinical intervention studies to test the hypothesis that HMOs contribute to the beneficial effect of human milk feeding.
NEC is one of the most common and fatal gastrointestinal disorders in preterm infants [25]. About 5% of all very-low- birth-weight (VLBW) infants develop NEC with a mortality rate of over 25%. While formula-fed infants are at a 6- to 10-fold higher risk to develop NEC [26], the protective mechanisms of feeding human milk remain poorly understood. In contrast to formula, human milk is an
abundant source of HMOs. In addition, the NEC-preventing effect of human donor milk persists even after pasteurization, a process that destroys and inactivates many human milk bioactives, but keeps HMOs intact and active. Based on these observations we hypothesized that HMOs contribute to the lower NEC incidence in human milk-fed infants, and tested the hypothesis in a neonatal rat model [27]. Newborn rat pups were either left with the dam to serve as “breast-fed” control or removed from the dam and received formula by oral gavage. While all of the dam-fed pups survived the first 4 days of life, only ~75% of the formula-fed pups survived. However, 95% of the pups survived when they received a formula that was supplemented with HMOs that were isolated from pooled human milk. This significant increase in survival coincided with decreased pathological observations, both macroscopically as well as microscopically. We then applied a multidimensional chromatography approach to separate the different HMOs first by charge and later by size and identified one specific HMO, disialyllacto-N-tetraose (DSLNT), which contains two Neu5Ac, to be responsible for the beneficial effects. Enzymatic removal of just one of the Neu5Ac led to a complete loss of function, indicating that the effect is highly structure-specific [27].
While the data was encouraging, the validity of available preclinical NEC models in rodents or piglets is limited [28]. Animals are exposed to external hypoxic and/or hypothermic insults that are rather artificial, and the use of animals itself is a limitation due to interspecies differences in gastrointestinal development, anatomy and physiology. Thus, advancing a potential therapeutic like DSLNT from controversial preclinical models to clinical treatment trials carried a tremendous risk of failure. To help close the gap between animal models and clinical intervention studies, we used an intermediate approach and conducted a multicenter clinical prospective cohort study with mothers and their VLBW infants fed predominantly human milk [29]. The study was based on the observation that some infants still develop NEC despite receiving predominantly human milk. Since HMO composition varies between women it led us to hypothesize that infants who develop NEC received milk with less DSLNT than infants who do not develop NEC. We recruited 200 mothers and their very low-birth-weight infants that were predominantly human milk-fed. We analyzed HMO composition in human milk fed to infants over the first 28 days post partum, matched each NEC case with five controls, and used logistic regression and generalized estimating equation to show that DSLNT concentrations were significantly lower in almost all milk samples in all 8 NEC cases when compared to controls. In fact, DSLNT abundance could identify NEC cases prior to onset. Aggregate assessment of DSLNT over multiple days enhanced the separation of NEC cases and control subjects, making DSLNT content in human milk a potential non-invasive marker to identify infants at risk of developing NEC.
While the cohort association data alone would raise questions about cause-and-effect, the combination with data from the preclinical model substantially increases the confidence that the observed effects are indeed due DSLNT, lowering the risk threshold for a clinical intervention study to fail.
Overall, the NEC project is an example for how the combination of preclinical data and clinical cohort data can inform clinical intervention studies (Figure 3). A similar approach has been used in the past to relate HMOs to a reduction of Campylobacter jejuni infection and diarrhea [21,30] and is currently used to study the effects of HMOs on infant body composition and childhood obesity risk as well as on allergies and asthma risk.
What we’ve learnt so far from this combined approach is that sometimes specific HMOs are effective and those effects are usually highly structure-specific and dose-dependent. The underlying mechanisms are likely mediated by specific receptors on host (infant) tissues or on microbes. In other cases, a combination of different HMOs in specific ratios to each other is required to be effective. The underlying mechanisms are likely mediated indirectly through shaping microbial communities or directly through a coordinated interaction of different HMOs with multiple different receptors or even different cell types, for example in the immune system. In situations where relative abundances of many different HMOs matter more than individual HMOs alone, it raises the question whether or not a woman’s characteristic HMO composition has evolved to match her own infant’s specific needs. In that case, HMOs can be seen as another example of personalized nutrition early on in life, which comes in addition to other personalized components of human milk like antibodies, milk microbiota, immune cells, and progenitor cells. In fact, the personalized complexity of HMOs provides yet another powerful reason to protect, promote, and support breast-feeding.
References
1. Bode L: Human milk oligosaccharides: Every baby needs a sugar mama. Glycobiology. 2012;22(9):1147-62.
2. Kobata A: Structures and application of oligosaccharides in human milk. Proc Jpn Acad Ser B Phys Biol Sci. 2010;86(7):731-747.
3. Chaturvedi P, Warren CD, Altaye M, Morrow AL, Ruiz-Palacios G, Pickering, LK, Newburg DS: Fucosylated human milk oligosaccharides vary between individuals and over the course of lactation. Glycobiology. 2001;11:365–372.
4. Stahl B, Thurl S, Henker J, Siegel M, Finke B, Sawatzki G: Detection of four human milk groups with respect to Lewis-blood-group-dependent oligosaccharides by serologic and chromatographic analysis. Adv Exp Med Biol 2001;501:299–306.
5. Kumazaki T, Yoshida A: Biochemical evidence that secretor gene, Se, is a structural gene encoding a specific fucosyltransferase. Proc Natl Acad Sci USA 1984;81:4193–4197.
6. Johnson PH, Watkins WM: Purification of the Lewis blood-group gene associated α-3/4-fucosyltransferase from human milk: An enzyme transferring fucose primarily to Type 1 and lactose-based oligosaccharide chains. Glycoconj J 1992;9:241–249.
7. Subbarao P, Anand SS, Becker AB, Befus AD, Brauer M, Brook JR, Denburg JA, HayGlass KT, Kobor MS, Kollmann TR, Kozyrskyj AL, Lou WY, Mandhane PJ, Miller GE, Moraes TJ, Pare PD, Scott JA, Takaro TK, Turvey SE, Duncan JM, Lefebvre DL, Sears MR; CHILD Study investigators: The Canadian Healthy Infant Longitudinal Development (CHILD) Study: examining developmental origins of allergy and asthma. Thorax 2015;70(10):998-1000.
8. Gnoth MJ, Kunz C, Kinne-Saffran E, Rudloff S: Human milk oligosaccharides are minimally digested in vitro. J Nutr. 2000;130:3014–3020.
9. Engfer MB, Stahl B, Finke B, Sawatzki G, Daniel H: Human milk oligosaccharides are resistant to enzymatic hydrolysis in the upper gastrointestinal tract. Am J Clin Nutr. 2000;71:1589–1596.
10. Ruhaak LR, Stroble C, Underwood MA, Lebrilla CB: Detection of milk oligosaccharides in plasma of infants. Anal Bioanal Chem. 2014;406(24):5775-84.
11. Goehring KC, Kennedy AD, Prieto PA, Buck RH: Direct evidence for the presence of human milk oligosaccharides in the circulation of breastfed infants. PLoS One. 2014;9(7):e101692.
12. Rudloff S, Obermeier S, Borsch C, Pohlentz G, Hartmann R, Brosicke H, et al.: Incorporation of orally applied (13)C-galactose into milk lactose and oligosaccharides. Glycobiology. 2006;16(6):477-87.
13. Dotz V, Rudloff S, Blank D, Lochnit G, Geyer R, Kunz C: 13C-labeled oligosaccharides in breastfed infants’ urine: individual-, structure- and time-dependent differences in the excretion. Glycobiology. 2014;24(2):185-194.
14. German JB, Freeman SL, Lebrilla CB, Mills DA: Human milk oligosaccharides: evolution, structures and bioselectivity as substrates for intestinal bacteria. Nestle Nutr Workshop Ser Pediatr Program. 2008;62:205-18.
15. Sela DA1, Chapman J, Adeuya A, Kim JH, Chen F, Whitehead TR, Lapidus A, Rokhsar DS, Lebrilla CB, German JB, Price NP, Richardson PM, Mills DA: The genome sequence of Bifidobacterium longum subsp. infantis reveals adaptations for milk utilization within the infant microbiome. Proc Natl Acad Sci U S A. 2008;105(48):18964-9.
16. Marcobal A, Barboza M, Sonnenburg ED, Pudlo N, Martens EC, Desai P, Lebrilla CB, Weimer BC, Mills DA, German JB, Sonnenburg JL: Bacteroides in the infant gut consume milk oligosaccharides via mucus-utilization pathways. Cell Host Microbe. 2011;10(5):507-14.
17. Lin AE, Autran CA, Szyszka A, Escajadillo T, Huang M, Godula K, Prudden AR, Boons GJ, Lewis AL, Doran KS, Nizet V, Bode L: Human milk oligosaccharides inhibit growth of group B Streptococcus. J Biol Chem. 2017;292(27):11243-11249.
18. Edwards MS: Issues of antimicrobial resistance in group B streptococcus in the era of intrapartum antibiotic prophylaxis. Semin Pediatr Infect Dis. 2006;17(3):149-52.
19. Heath PT, Schuchat A: Perinatal group B streptococcal disease. Best Pract Res Clin Obstet Gynaecol. 2007;21(3):411-24.
20. Thigpen MC, Whitney CG, Messonnier NE, Zell ER, Lynfield R, Hadler JL, et al.: Bacterial meningitis in the United States, 1998-2007. N Engl J Med. 2011;364(21):2016-25.
21. Ruiz-Palacios GM, Cervantes LE, Ramos P, Chavez-Munguia B, Newburg DS: Campylobacter jejuni binds intestinal H (O) antigen (Fucα1,2Galβ1,4GlcNAc), and fucosyloligosaccharides of human milk inhibit its binding and infection. J Biol Chem. 2003;278:14112–14120.
22. Manthey CF, Autran CA, Eckmann L, Bode L: Human Milk Oligosaccharides protect against enteropathogenic E. coli attachment in vitro and colonization in suckling mice. J Pediatr Gastroenterol Nutr. 2014;58(2):167-70.
23. Jantscher-Krenn E, Lauwaet T, Bliss LA, Reed SL, Gillin FD, Bode L:. Human Milk Oligosaccharides reduce Entamoeba histolytica attachment and cytotoxicity in vitro. Br J Nutr. 2012;108(10):1839-46
24. Lin AE, Autran CA, Espanola SD, Bode L, Nizet V: Human Milk Oligosaccharides Protect Bladder Epithelial Cells Against Uropathogenic Escherichia coli Invasion and Cytotoxicity. J Infect Dis. 2014;209(3):389-98
25. Neu J, Walker WA:. Necrotizing enterocolitis. N Engl J Med. 2011;364(3):255-264.
26. Lucas A, Cole TJ. Breast milk and neonatal necrotising enterocolitis. Lancet. 1990;336:1519-23.
27. Jantscher-Krenn E, Zherebtsov M, Nissan C, Goth K, Guner YS, Naidu N, Choudhury BP, Grishin AV, Ford HR, Bode L: The human milk oligosaccharides disialyllacotse-N- tetraose prevents Necrotizing Enterocolitis in neonatal rats. Gut 2012;61:1417-25.
28. Tanner SM, Berryhill TF, Ellenburg JL, Jilling T, Cleveland DS, Lorenz RG, Martin CA: Pathogenesis of necrotizing enterocolitis: modeling the innate immune response. Am J Pathol. 2015;185(1):4-16.
29. Autran CA, Kellman BP, Kim JH, Asztalos E, Blood AB, Hamilton Spence EC, Patel AL, Hou J, Lewis NE, Bode L: Human milk oligosaccharide composition predicts risk of necrotizing enterocolitis in preterm infants. Gut 2017;(In Press) doi: 10.1136/gutjnl-2016-312819.
30. Morrow AL, Ruiz-Palacios GM, Altaye M, Jiang X, Guerrero ML, Meinzen-Derr JK, Farkas T, Chaturvedi P, Pickering LK, Newburg DS: Human milk oligosaccharides are associated with protection against diarrhea in breast-fed infants. J Pediatr. 2004;145:297–303
