Human Milk Oligosaccharides: Factors Affecting Their Composition and Their Physiological Significance
Abstract
Human milk oligosaccharides (HMOs) are elongations of the milk sugar lactose by galactose, N-acetylglucosamine, fucose; and sialic acid. The HMO composition of breast milk is strongly influenced by polymorphisms of the maternal fucosyltransferases, FUT2 and FUT3, and by the stage of lactation. Clinical observational studies with breastfed infant- mother dyads associate specific HMOs with infant gut microbiota, morbidity, infectious diarrhea, and allergies. Observational and basic research data suggest that HMOs influence the establishment of early-life microbiota and mucosal immunity and inhibit pathogens, thereby contributing to protection from infections. Clinical intervention trials with infant formula supplemented with the single HMO, 2′-fucosyllactose (2′FL), or with 2 HMOs, 2′FL and lacto-N-neotetraose (LNnT), demonstrated that they allow for age-appropriate growth and are well tolerated. A priori defined exploratory outcomes related feeding an infant formula with 2 HMOs to fewer reported illnesses of the lower respiratory tract and reduced need for antibiotics during the first year of life compared to feeding a control formula. In parallel, early-life microbiota composition shifted towards that of breastfed infants. Together, HMOs likely contribute to immune protection in part through their effect on early-life gut microbiota, findings that warrant further clinical research to improve our understanding of HMO biology and significance for infant nutrition.
Introduction
What are human milk oligosaccharides (HMOs)? What is their importance for infant nutrition? These questions have intrigued scientists and pediatricians alike for over a century. Advances in analytics as well as large-scale synthesis technologies stimulated great progress in recent years. These provided the ma- terials and tools that enabled the detailed and accurate measurement of HMO quality and quantity, and the study of HMOs in basic research models, and through clinical observational studies and intervention trials.
“HMOs are not HMOs,” meaning that one specific HMO is not equal to another HMO, especially when considering structure-function relationships. Chemically, HMOs are elongations of the milk-specific sugar lactose in different linkages by one or a combination of the following monosaccharides: L-fucose (Fuc), D-galactose (Gal), N-acetyl-D-glucosamine (GlcNAc), and N-acetylneuraminic acid (sialic acid). Gal and GlcNAc generally elongate lactose as a disaccharide Gal-GlcNAc. The numerous and diverse HMOs produced might be categorized by specific structural features brought about by different glycosyltransferases involved in their synthesis. However, many HMOs combine different structural features.
Breast milk, the recommended and naturally adapted nutrition for infants, is associated with a reduced risk for infection-related illnesses and possibly for diabetes and overweight, while the situation for allergies is less clear [1]. This suggests that breast-milk-specific components such as HMOs and other bioactives may contribute to such benefits.
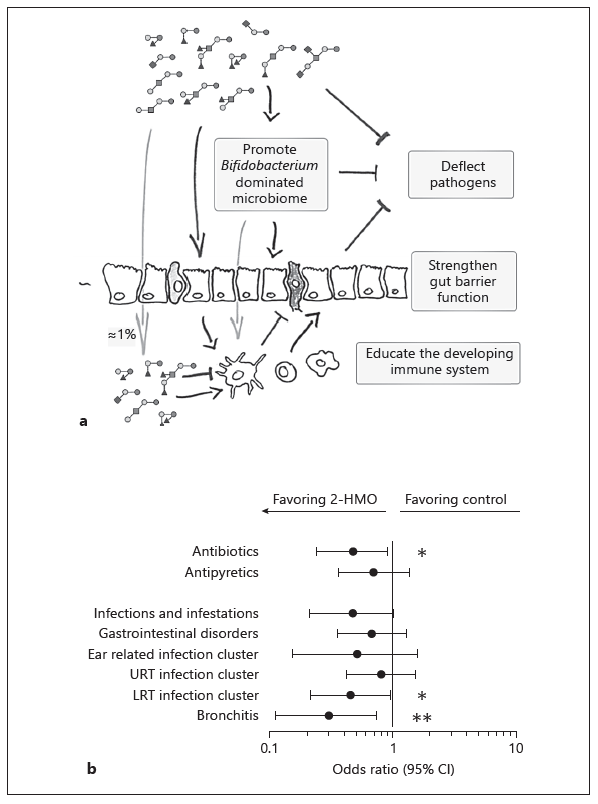
Due to their structural similarity with mucosal glycans and their nondigestible nature, HMOs expectedly affect numerous glycan-mediated processes like the colonization of the early-life microbiota and the infectivity of pathogens (Fig. 1a). Based on clinical observational and basic research data, HMOs act in a structure-function-specific way helping the (i) establishment of a mucous-adapted microbiome, (ii) resistance to pathogens, and (iii) reactivity of the mucosal barrier and immunity, thereby contributing to immune protection.
Here, we briefly review genetic and environmental factors affecting HMO composition in breast milk and the physiological role of HMOs as supported by clinical observation studies, preclinical research on mode of action, and insights from clinical intervention trials.
Maternal Glycosyltransferase Polymorphisms Affect HMO Composition
HMOs resemble the blood group antigens and further sialylated glycans that cover the human mucosa. The same glycosyltransferases are generally involved in the synthesis of mucosal cell glycans and mammary gland-expressed HMOs. The fucosyltransferases FUT2 (secretor gene) and FUT3 (Lewis gene) are the best described due to their natural polymorphisms in humans [2] (Fig. 2a). Specific genetic polymorphisms abolish their respective enzyme activity. Thus, specific HMO structures that depend on FUT2 or FUT3 can be identified. FUT2- dependent HMOs all contain α1,2-linked fucose, for example 2′-fucosyllactose (2′FL), lactodifucosyllactose (LDFT), and lacto-N-fucosylpentose (LNFP)-I. In- terestingly, trace amounts of 2′FL were found in breast milk of presumed FUT2-negative mothers in Asian populations [3, 4], indicating that the nature of these inactivating polymorphisms and thus the HMO profile may be population specific [5]. Typical FUT3-dependent HMOs are LNFP-II, lacto-N-difucosylhexose (LNDFH)-I containing α1,4-linked fucose, and to a lesser extent 3-fucosyllactose (3FL) and LDFT containing α1,3-linked fucose. In breast milk that does not contain any detectable LNFP-II, reduced amounts of HMOs with α1,3-linked fucose on glucose and increased amounts of those with an α1,3-linked Fuc on GlcNAc are found. Hence, another FUT (e.g., FUT4, FUT5, FUT6, FUT7, or FUT9) is also involved in HMO formation with an α1,3-linked Fuc on GlcNAc and glucose.
The absence of a functional FUT2 or FUT2 and FUT3 affects the concentra- tion of total HMOs in milk when expressed as the sum of all quantified HMOs [2] (Fig. 2). While some HMOs increase when FUT2 is missing (e.g., LNnT and 3FL), in the absence of fucosylation additional larger nonfucosylated HMOs might also be produced.
To date, no common genetic polymorphisms for sialylated HMOs have been described, indicating that if inactivating polymorphisms in sialyltransferase genes exist, they are extremely rare. From mouse studies, the sialyltransferases ST6Gal1 and ST3Gal4 are involved in the synthesis of 6′-sialyllactose (6′SL) and 3′-sialyllactose (3′SL), respectively, with a further sialyltransferase, probably ST3Gal1, also making 3′SL [6].
Another mechanism affecting HMO composition is probably the donor and acceptor substrate availability, as suggested by the increase in 3FL when the ma- jor fucosyl-HMO 2′FL decreases in concentration [3].
Interestingly, HMO concentrations change during the stage of lactation with different HMOs showing different dynamics [3]. HMOs like 6′SL or LNnT decrease more rapidly during the first weeks of lactation, while 2′FL and 3′SL, for example, decrease more slowly over a longer time period, and again others, like 3FL, actually increase in concentration with time of lactation (Fig. 2b).
Such compositional changes due to the genetic background of mothers and stage of lactation can confound observations relating HMOs to clinical parameters in the breastfed infants and, therefore, need to be considered.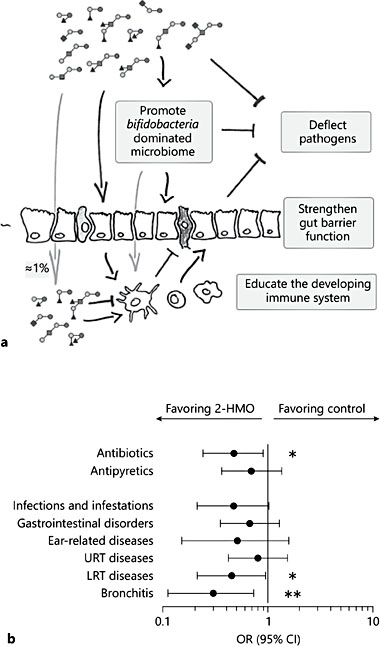
Fig. 1. Illustration of the different biological functions of human milk oligosaccharides (HMOs). a Risk for infection-related illnesses and medication use in infants fed a formula supplemented with 2 HMOs (redrawn from Puccio et al. [49]). b Upper (URT) and lower respiratory tract (LRT) infections and other diseases as well as medication use were re- ported by parents and verified by a study physician. ORs with 95% CIs are shown based on percent of infants with at least 1 event at 1 year of age (Fisher’s exact test: * p < 0.05,** p < 0.001) (replotted from Ferrer-Admetlla et al. [5]).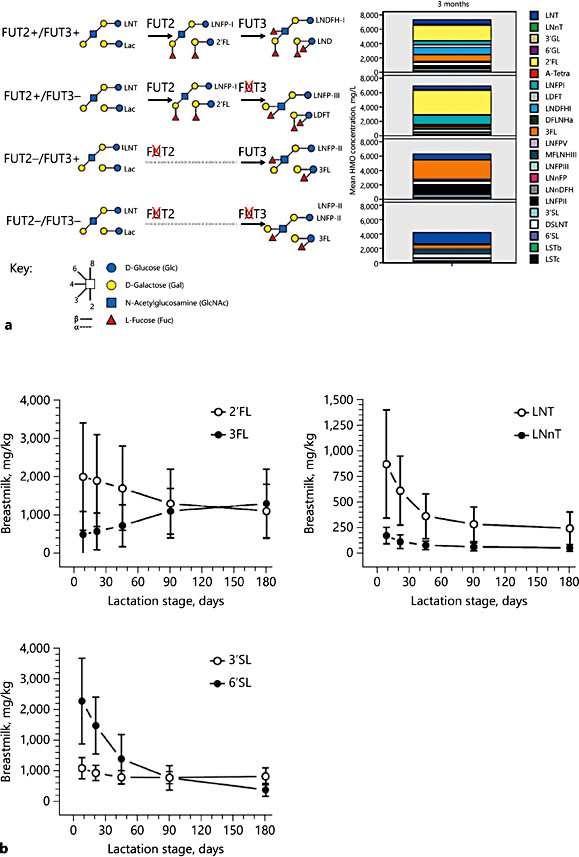
Fig. 2. Human milk oligosaccharide (HMO) composition 3 months postpartum by FUT2 and FUT3 status with schematic illustration of typical HMO in one or the other group (a). HMO dynamics at different stages of lactation (replotted from Austin et al. [3]) depicting mean concentrations with standard deviations (b).
HMO Composition and Maternal Diet, Gestational Age, and Physiological State of the Infant
HMO concentrations in colostrum, transitional milk, and mature milk seem not to change between mothers giving birth to preterm (n = 18; gestational age<37 weeks) and term (n = 14; gestational age ≥37 weeks) infants [2]. Further, fucosylated and sialylated HMOs were reported to be similar between preterm and term milk, although preterm milk seemed more variable in the expression of fucosylated HMOs [7].
Today, we do not know whether and how maternal diet might influence HMO composition. A recent observational study including 33 breastfeeding mothers and their infants from the Gambia, Africa, reported a significantly higher HMO content in milk at 20 weeks of lactation in the dry season (n = 21) than the wet season (n = 12) [8]. The authors propose a possible link to the high- er energy intake during the dry season. In 2 other African mother-infant cohorts from Malawi (n = 88 and n = 215), total HMOs and also sialyl- and fucosyl-HMOs were lower 6 months postpartum in the breast milk of mothers having severely stunted infants compared to those with normal-size infants [9]. These studies suggest that maternal nutritional and health status may affect HMO composition.
By analogy, higher maternal body mass index and gestational weight gain, which generally reflects an altered metabolic physiology, might affect HMO composition. Studies to this end are currently ongoing [10; Binia et al.: abstract at FASEB Science Research Conferences in 2017]. Suitable studies are warranted to investigate possible alterations in HMO composition due to maternal energy and specific nutrient intake.
The HMO Composition Is Associated with the Gut Microbiota in Infants
The early-life microbiome has a major impact on the developing immune system, itself being an important element by providing pathogen colonization resistance, for example. Interestingly, the establishing intestinal microbiota also contributes, via an innate lymphoid cell-mediated process, to improved protection against respiratory tract infection [11]. The pioneers of human milk and breastfeeding research observed a strong link between breastfeeding and immune protection to infectious morbidity and mortality. Breastfed infants were recognized to harbor an early gut microbiota domi- nated by bifidobacteria, not seen in formula-fed infants, and a human-milk- specific “bifidofactor” was identified in the HMO fraction of breast milk [12].
From research on early-life microbiota, we know that bifidobacteria can utilize and grow on different individual HMOs in a strain-specific way [13, 14]. Several studies observed an increased bacterial metabolic activity upon growth on HMOs, exemplified by the formation of the short-chain fatty acid acetate [15, 16]. Noteworthy, numerous potentially pathogenic bacteria from the Enterobacteriaceae group were shown not to grow on individual HMOs as the sole carbon source [17], while growth of other pathogens, like Streptococcus agalactiae (group B Streptococcus, GBS) was shown to be inhibited by HMOs [18, 19].
Recently, LNnT in breast milk was associated with Bifidobacterium longum ssp. infantis abundance [8]. In bi-associated gnotobiotic mice harboring only 1 Bacteroides and 1 B. longum ssp. infantis strain, LNnT lead to bifidobacteria dominance although both bacteria could actually use LNnT in vitro [20]. In gnotobiotic mice humanized with 7 human microbes, B. longum ssp. infantis also showed higher abundance when these mice were fed 2′FL combined with LNnT as compared to LNnT alone [Sprenger et al., unpubl. observation], although B. longum ssp. infantis is able to grow on many different HMOs, including LNnT, as substrate [13].
Genomic and glycomic analyses in infants provided further evidence for a role of HMOs in shaping the early infant gut microbiome, revealing associations between individual HMOs and bacterial genera in infant stool [21–23]. A Bifidobacterium-dominated gut microbiota in breastfed infants (n = 105) at 4 months of age was associated with breast milk containing FUT2-HMOs [24]. The FUT2 status of the infants and its possible confounding effects on the infant microbiota profile were not assessed, despite earlier data proposing the FUT2 status itself can influence the gut microbiota at least in adults [25]. In another cohort, the analysis of a relatively small subgroup of infants exclusively breastfed for 4 months (n = 14) showed an association of maternal FUT2-positive status with higher Bifidobacterium abundance up to 2–3 years of age [26]. However, no statistically significant HMO effects on global Bifidobacterium shifts were reported in another recent study of 33 Gambian mothers and infants [8], while the abundance of individual bifidobacteria like B. longum ssp. infantis still correlated with LNnT concentrations in breast milk. These first reports reveal the need for larger observational studies of similar design, including comprehensive HMO analysis of breast milk and infant FUT2 phenotyping to gain a more robust understanding of the link between HMO and infant gut microbiome composition.
Today, clinical observations in conjunction with basic research data suggest that FUT2-HMOs, like 2′FL and LNFP-I, but likely also other non-FUT2-dependent HMOs, like LNnT for example, are involved in the establishment of a Bifidobacterium-dominated early-life gut microbiota. In vitro studies help to understand HMO-related microbial metabolic capacities and strain specificities, while animal and human observational studies indicate that the interaction between bacteria and the gut mucosa reflect a more complex picture. Hence, with infant health in mind, it is central to gain a better understanding of HMO effects on the microbiome dynamics in their natural ecosystem through a holistic and ecology-inspired approach.
HMO Composition Is Linked to Infection Risk in Infants
HMOs were studied in relation with infectious diarrhea incidence in a cohort of Mexican mothers and infants (n = 93) [27, 28]. Higher breast milk concentrations of α1,2-fucosylated HMOs were associated with a lower incidence of all-cause moderate-to-severe diarrhea. The most frequently identified cause of diarrhea in the cohort was Campylobacter jejuni followed by calicivirus and enteropathogenic Escherichia coli. Specifically, higher concentrations of 2′FL and LNFP-I in breast milk correlated with a lower incidence of C. jejuni and calici- virus diarrhea, respectively. These observations during the breastfeeding period did not persist in the period after breastfeeding, indicating a possible transient HMO effect in the protection from infectious diarrhea. This fits their presumed role as anti-adhesive antimicrobials. Experimental data from preclinical models also show protective effects of 2′FL from C. jejuni [29] and aggregating invasive E. coli [30]. From these data, 2′FL and other FUT2-HMOs seem to act as soluble ligands blocking C. jejuni from adhering to gut epithelial cells, while the protection from E. coli might rather be due to an anti-inflammatory effect, possibly combined with the modulation of the gut microbiota composition.
Glycans containing α1,2-linked Fuc expressed on epithelial cells of FUT2- positive infants could act as receptors for pathogen binding, conferring a risk to specific infectious diseases for this population [31]. Genetic studies have shown that infants and children with a nonfunctional FUT2 gene have strain-specific protection against norovirus and rotavirus [32, 33]. For specific rotavirus strains, susceptibility depends on FUT2 but also on FUT3 status [34]. Experimentally, infectivity of some rotavirus strains was reduced by the FUT2 HMO 2′FL, while other viral strains were affected by sialylated HMO, namely 3′SL and 6′SL [35]. Similarly, 2′FL also bound to specific norovirus strains [36].
Besides interfering with pathogen attachment to the host mucosa, HMOs were recently reported to exert bacterial-growth-inhibitory activities on pathogenic GBS [18, 19, 37], a major cause of sepsis in preterm infants. Growth of GBS was specifically inhibited by LNT and LNFP-I, while sialylated HMOs or galactooli- gosaccharides (GOS) had no effect [19]. Experimental data suggest a putative gly- cosyltransferase of GBS to be involved [19]. Possibly pointing to a similar mechanism, HMOs from milk of a FUT2-negative mother were shown to have bacterio static properties via an alteration in biofilm formation [18]. In an observation study of 183 Gambian infant-mother pairs, FUT3-positive mothers were reported to be less likely carriers of GBS, as were their infants at birth [37]. Interestingly, infants of FUT3-positive mothers were also more likely to clear GBS colonization from birth to 2–3 months of age compared to infants of FUT3-negative mothers.
In a pilot study of 49 mother-infant pairs, higher breast milk concentrations of the FUT3-HMO LNFP-II at 2 weeks were associated with a lower risk of respiratory and gastrointestinal illnesses at 6 and 12 weeks in infants [38]. This association was no longer significant after the breastfeeding period. Similarly, in a nested case cohort study of 143 HIV-exposed uninfected children from Zambia, higher concentrations of fucosylated HMOs in breast milk 1 month postpartum related to a lower risk of mortality up to 2 years of age [39]. In another small mother-infant cohort from the Gambia (n = 33), higher relative breast milk concentrations of fucosylated HMO (sum of LNFP-I and LNFP-III) and concomitant lower relative abundance of LNT was associated with a lower risk of sickness up to 4 months of age [8].
For respiratory pathogens, direct HMO exposure would appear less evident, and thus any putative HMO-related protection may be mediated by the intestinal microbiome [11, 40]. Yet, experimentally, direct exposure of Streptococcus pneumoniae to LNnT and sialyl-LNnT and subsequent infection effectively blocked its colonization in the lung of a rabbit model [41]. In a cell-based assay, LNnT and 2′FL dose-dependently reduced influenza and respiratory syncytial virus concentrations within respiratory tract cells [42].
Observational studies together with findings from preclinical models have provided first evidence for an association between HMOs and the risk of infections, mostly in a structure-function-specific way. Mechanistically, HMOs may act through multiple functions, although preclinical models highlight specific individual functions. The current studies also provide directions to be considered in future observational studies, such as timing of milk sampling and breast milk intake, etiology of infections, quantitative versus categorical HMO analysis and finally mother and infant genetics.
HMO Composition Might Be Linked to Allergy in Infants
Numerous environmental, including nutrition, and genetic factors affect allergies. Among them are breast milk bioactives and possibly HMOs. In a cohort of 266 Finnish mother-infant pairs with a hereditary allergy risk, 2′FL concentrations in early breast milk associated with a lower risk to manifest IgE-associated eczema at 2 years of age only in C-section born infants [43]. This observation suggests that 2′FL may influence IgE-associated eczema through the modulation of the early-life gut microbiota, known to be different in C-section-born infants compared to vaginal-born infants. A possible relation of HMOs with cow milk allergy (CMA) was studied in another cohort of 39 mothers with infants who developed CMA by 18 months of age and 41 mothers with infants without CMA [44]. An association was seen between the milk concentration of several indi- vidual HMOs [LNFP-III, 6′SL, LNFP-I, and DSLNT (DiSialyllacto-N-tetraos)] and HMO clusters with reduced risk of CMA, with LNFP-III providing the strongest association. Breast milk sampling varied over the first 6 months after birth and this was taken into account in the statistical analysis, because HMO concentrations change dramatically during this period. Mechanistically, the authors speculate that LNFP-III might act on the immune system via dendritic cells and DC-SIGN (Dendritic Cell-Specific Intercellular adhesion molecule- 3-Grabbing Non-integrin). In a preclinical food allergy model, 2′FL and 6′SL were tested and both reduced symptoms involving mast cell activity [45].
The observational studies to date have their limitations, but still provide valuable preliminary data on possible relationships between specific HMOs and risk of allergies. To appreciate such a proposed link requires replication in larger cohorts with harmonized milk sampling, stratification for mode of delivery, and evaluation of infant FUT2 and FUT3 genotypes.
Insight from Clinical Intervention Trials with Specific HMOs
Recent progress in industrial biotechnology has made available few individual HMOs, namely 2′FL and LNnT. Preclinical safety toxicity tests established their safety, and both obtained approval as novel foods in the European Union and were generally recognized as safe in the USA.
In adults, both 2′FL and LNnT were studied alone or in combination at different doses from 5 to 20 g/day in a placebo-controlled, blinded, randomized trial (n = 100). Both HMOs were well tolerated and increased bifidobacterial abundance [46].
In infants, 2 placebo-controlled, blinded, randomized, clinical intervention trials showed the growth safety and tolerance of 2′FL combined with either GOS or fructooligosaccharides [47, 48; Kajizer et al., unpubl.]. Infants fed with an infant formula supplemented with 2′FL (0.2 or 1 g/L) combined with GOS or GOS alone showed similar growth as breastfed infants up to 4 months of age (n = 314). In a subgroup of infants, immune markers were measured in plasma at baseline and upon stimulation of blood cells with respiratory syncytial virus. Globally the immune profile resembled that of breastfed infants when the infant formula was supplemented with 2′FL at the lower or higher dose [48]. Another randomized controlled infant trial showed that an infant starter formula supplemented with 2 HMO, 2′FL, and LNnT (n = 88) allowed for age-appropriate growth of term born infants and was well tolerated when compared to the same infant formula without HMO (n = 87) [49]. Interestingly, secondary exploratory findings showed an association between feeding the 2-HMO infant formula and less-re- ported lower respiratory tract illnesses and medication use (especially antibiotics and antipyretics) during the first year of life and beyond the 6-month feeding period. At 3 months, the global microbiota profile shifted in the 2-HMO-formula-fed infants away from the control-formula-fed infants and towards that ob- served in breastfed reference infants. This shift was mainly due to increases in Bifidobacterium concomitant with decreases in Escherichia and Peptostrepto- coccaceae [50]. A significantly higher number of infants who were fed the 2-HMO-supplemented formula showed a microbiota community structure typical for breastfed infants compared to control-formula-fed infants, who had primarily a different microbiota community structure. Interestingly, infants with a microbiota community structure typical for control-formula-fed infants had a 2 times higher risk to use antibiotics during the first year of life than those with a microbiota community typical for breastfed infants [51].
These first clinical intervention trials with specific HMOs demonstrate their growth safety and digestive tolerance. Additionally, as suggested from basic re- search and observational data, 2′FL and LNnT might contribute to the protection from infection-related illnesses and reduce the need for antibiotics, possibly through the modulation of the establishing early-life gut microbiota.
Conclusion
HMO composition is affected most notably by the maternal FUT2 and FUT3 status. This is likely due to an evolutionary selective pressure imposed by pathogens or the microbiome at large. Stage of lactation alters HMO composition possibly indicating different infant needs at different extrauterine developmental stages. However, giving birth to a preterm or term infant, who are at different developmental stages, seems not to affect the HMO composition of breast milk. Clinical observations corroborated by preclinical data and clinical intervention trials support a role for specific HMOs in immune protection, primarily from infection-related morbidity and use of antibiotics. Further clinical studies, well- designed observational studies, and especially placebo-controlled interventions are warranted to further substantiate and grow our understanding of the HMO biology and significance for infant nutrition.
References
- 1 Victora CG, Bahl R, Barros AJ, et al: Breast- feeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet 2016; 387:475–490.
- 2 Kunz C, Meyer C, Collado MC, et al: Influence of gestational age, secretor, and Lewis blood group status on the oligosaccharide content of human milk. J Pediatr Gastroenterol Nutr 2017;64:789–798.
- 3 Austin S, De Castro CA, Bénet T, et al: Temporal Change of the content of 10 oligosaccharides in the milk of Chinese urban mothers. Nutrients 2016;8:1–22.
- 4 Sprenger N, Lee LY, De Castro CA, et al: Longitudinal change of selected human milk oligosaccharides and association to infants’ growth: an observatory, single center, longtudinal cohort study. PLoS One 2017; 12:e0171814.
- 5 Ferrer-Admetlla A, Sikora M, Laayouni H, et al: A natural history of FUT2 polymorphism in humans. Mol Biol Evol 2009;26:1993– 2003.
- 6Fuhrer A, Sprenger N, Kurakevich E, et al: Milk sialyllactose influences colitis in mice through selective intestinal bacterial colonization. J Exp Med 2010;207:2843–2854.
- 7 De Leoz ML, Gaerlan SC, Strum JS, et al: Lac- to-N-tetraose, fucosylation, and secretor status are highly variable in human milk oligosaccharides from women delivering preterm. J Proteome Res 2012;11:4662–4672.
- 8 Davis JC, Lewis ZT, Krishnan S, et al: Growth and morbidity of Gambian infants are influenced by maternal milk oligosaccharides and infant gut microbiota. Sci Rep 2017;7:40466.
- 9 Charbonneau MR, O'Donnell D, Blanton LV, et al: Sialylated milk oligosaccharides promote microbiota-dependent growth in models of infant undernutrition. Cell 2016;164: 859–871.
- 10 McGuire MK, Meehan CL, McGuire MA, et al: What’s normal? Oligosaccharide concentrations and profiles in milk produced by healthy women vary geographically. Am J Clin Nutr 2017;105:1086–1100.
- 11 Gray J, Oehrle K, Worthen G, et al: Intestinal commensal bacteria mediate lung mucosal immunity and promote resistance of new- born mice to infection. Sci Transl Med 2017; 9:1–13.
- 12 Kunz C: Historical aspects of human milk oligosaccharides. Adv Nutr 2012;3:430S– 439S.
- 13 Sela DA, Mills DA: Nursing our microbiota: molecular linkages between bifidobacteria and milk oligosaccharides. Trends Microbiol 2010;18:298–307.
- 14 Matsuki T, Yahagi K, Mori H, et al: A key genetic factor for fucosyllactose utilization affects infant gut microbiota development. Nat Commun 2016;7:11939–11951.
- 15 Li M, Bauer LL, Chen X, et al: Microbial composition and in vitro fermentation pat- terns of human milk oligosaccharides and prebiotics differ between formula-fed and sow-reared piglets. J Nutr 2012;142:681–689.
- 16 Yu ZT, Chen C, Kling DE, et al: The principal fucosylated oligosaccharides of human milk exhibit prebiotic properties on cultured infant microbiota. Glycobiology 2013;23:169– 177.
- 17 Hoeflinger JL, Davis SR, Chow J, Miller MJ: In vitro impact of human milk oligosaccha- rides on Enterobacteriaceae growth. J Agric Food Chem 2015;63:3295–3302.
- 18 Ackerman DL, Doster RS, Weitkamp JH, et al: Human milk oligosaccharides exhibit anti- microbial and antibiofilm properties against group B Streptococcus. ACS Infect Dis 2017;3: 595–605.
- 19 Lin AE, Autran CA, Szyszka A, et al: Human milk oligosaccharides inhibit growth of group B Streptococcus. J Biol Chem 2017;292: 11243–11249.
- 20 Marcobal A, Barboza M, Sonnenburg ED, et al: Bacteroides in the infant gut consume milk oligosaccharides via mucus-utilization pathways. Cell Host Microbe 2011;10:507– 514.
- 21 De Leoz ML, Wu S, Strum JS, et al: A quanti- tative and comprehensive method to analyze human milk oligosaccharide structures in the urine and feces of infants. Anal Bioanal Chem2013;405:4089–4105.
- 22 Underwood MA, Gaerlan S, De Leoz ML, et al: Human milk oligosaccharides in premature infants: absorption, excretion, and influence on the intestinal microbiota. Pediatr Res 2015;78:670–677.
- 23 Wang M, Li M, Wu S, et al: Fecal microbiota composition of breast-fed infants is correlated with human milk oligosaccharides consumed. J Pediatr Gastroenterol Nutr 2015;60: 825–833.
- 24Lewis ZT, Totten SM, Smilowitz JT, et al: Maternal fucosyltransferase 2 status affects the gut bifidobacterial communities of breastfed infants. Microbiome 2015;3:13–34.
- 25 Wacklin P, Mäkivuokko H, Alakulppi N, et al: Secretor genotype (FUT2 gene) is strongly associated with the composition of bifidobacteria in the human intestine. PLoS One 2011; 6:e20113.
- 26 Smith-Brown P, Morrison M, Krause L, Da- vies PS: Mothers secretor status affects development of childrens microbiota composition and function: a pilot study. PLoS One 2016; 11:e0161211.
- 27 Morrow AL, Ruiz-Palacios GM, Altaye M, et al: Human milk oligosaccharide blood group epitopes and innate immune protection against Campylobacter and calicivirus diarrhea in breastfed infants. Adv Exp Med Biol 2004;554:443–446.
- 28 Newburg DS, Ruiz-Palacios GM, Altaye M, et al: Innate protection conferred by fucosylated oligosaccharides of human milk against diarrhea in breastfed infants. Glycobiology 2004; 14:253–263.
- 29 Ruiz-Palacios GM, Cervantes LE, Ramos P, et al: Campylobacter jejuni binds intestinal H(O) antigen (Fucα1,2Galβ1,4GlcNAc),and fucosyloligosaccharides of human milk inhib- it its binding and infection. J Biol Chem 2003; 278:14112–14120.
- 30 He Y, Liu S, Kling DE, et al: The human milk oligosaccharide 2′-fucosyllactose modulates CD14 expression in human enterocytes, thereby attenuating LPS-induced inflammation. Gut 2016;65:33–46.
- 31 Le Pendu J: Histo-blood group antigen and human milk oligosaccharides: genetic polymorphism and risk of infectious diseases. Adv Exp Med Biol 2004;554:135–143.
- 32 Thorven M, Grahn A, Hedlund KO, et al: A homozygous nonsense mutation (428G–>A) in the human secretor (FUT2) gene provides resistance to symptomatic norovirus (GGII) infections. J Virol 2005;79:15351–15355.
- 33I mbert-Marcille BM, Barbé L, Dupé M, et al: A FUT2 gene common polymorphism deter- mines resistance to rotavirus A of the P[8] genotype. J Infect Dis 2014;209:1227–1230.
- 34 Nordgren J, Sharma S, Bucardo F, et al: Both Lewis and secretor status mediate susceptibility to rotavirus infections in a rotavirus geno-type-dependent manner. Clin Infect Dis 2014;59:1567–1573.
- 35 Laucirica DR, Triantis V, Schoemaker R, et al: Milk oligosaccharides inhibit human rota- virus infectivity in MA104 cells. J Nutr 2017; 147:1709–1714.
- 36 Koromyslova A, Tripathi S, Morozov V, et al: Human norovirus inhibition by a humanmilk oligosaccharide. Virology 2017;508:81– 89.
- 37 Andreas NJ, Al-Khalidi A, Jaiteh M, et al: Role of human milk oligosaccharides in group B Streptococcus colonisation. Clin Transl Immunology 2016;5:e99.
- 38 Stepans MB, Wilhelm SL, Hertzog M, et al: Early consumption of human milk oligosaccharides is inversely related to subsequent risk of respiratory and enteric disease in infants. Breastfeed Med 2006;1:207–215.
- 39 Kuhn L, Kim HY, Hsiao L, et al: Oligosaccha- ride composition of breast milk influences survival of uninfected children born to HIV- infected mothers in Lusaka, Zambia. J Nutr 2015;145:66–72.
- 40 Steed AL, Christophi GP, Kaiko GE, et al: The microbial metabolite desaminotyrosine protects from influenza through type I interferon.Science 2017;357:498–502.
- 41 Idanpaan-Heikkila I, Simon PM, Zopf D, et al: Oligosaccharides interfere with the establishment and progression of experimental pneumococcal pneumonia. J Infect Dis 1997; 176:704–712.
- 42 Duska-McEwen G, Senft AP, Ruetschilling TL, et al: Human milk oligosaccharides enhance innate immunity to respiratory syncytial virus and influenza in vitro. Food Nutr Sci 2014;5:1387–1398.
- 43 Sprenger N, Odenwald H, Kukkonen AK, et al: FUT2-dependent breast milk oligosaccharides and allergy at 2 and 5 years of age in infants with high hereditary allergy risk. Eur J Nutr 2017;56:1293–1301.
- 44 Seppo AE, Autran CA, Bode L, Järvinen KM: Human milk oligosaccharides and development of cow’s milk allergy in infants. J Allergy Clin Immunol 2017;139:708–711.
- 45 Castillo-Courtade L, Han S, Lee S, et al: Attenuation of food allergy symptoms following treatment with human milk oligosaccharides in a mouse model. Allergy 2015;70:1091–1102.
- 46 Elison E, Vigsnaes LK, Rindom Krogsgaard L, et al: Oral supplementation of healthy adults with 2′-O-fucosyllactose and lacto-N-neotetraose is well tolerated and shifts the intestinal microbiota. Br J Nutr 2016;116: 1356–1368.
- 47 Marriage BJ, Buck RH, Goehring KC, et al: Infants fed a lower calorie formula with 2′FL show growth and 2′FL uptake like breast-fed infants. J Pediatr Gastroenterol Nutr 2015;61: 649–658.
- 48 Goehring KC, Marriage BJ, Oliver JS, et al: Similar to those who are breastfed, infants fed a formula containing 2′-fucosyllactose have lower inflammatory cytokines in a randomized controlled trial. J Nutr 2016;146:2559– 2566.
- 49 Puccio G, Alliet P, Cajozzo C, et al: Effects of infant formula with human milk oligosaccharides on growth and morbidity: a randomized multicentertrial.JPediatrGastroenterol Nutr 2017;64:624–631.
- 50 Alliet P, Puccio G, Janssens E, et al: Term infant formula supplemented with human milk oligosaccharides (2′fucosyllactose and lacto- N-neotetraose) shifts stool microbiota and metabolic signatures closer to that of breast- fed infants. J Pediatr Gastroenterol Nutr 2016;63:S55.
- 51 Berger B, Grathwohl D, Alliet P, et al: Stool microbiota in term infants fed formula supplemented with synthetic human milk oligosaccharides is associated with reduced likelihood of medication (abstract). WCPGHAN, Montreal, 2016.