Human Milk MicroRNAs/Exosomes: Composition and Biological Effects
Abstract
Human milk contains a wide variety of bioactive components, including long-chain fatty acids, complex oligosaccharides, and bioactive proteins. More recently, it was discovered that breast milk also contains exosomes, i.e., microvesicles containing microRNAs (miR- NAs) with sizes of ∼22 nucleotides. Several of these miRNAs have been shown to origi- nate from the mammary gland, and many of them are involved in cellular development and immune function. Exosome-mediated transfer of miRNAs is a novel mechanism of genetic exchange between cells. It is therefore possible that exosomes in milk may survive digestion and deliver miRNAs to intestinal cells, or, if transferred into the blood stream, to cells in other tissues. In vitro work has shown that exosomes and their miRNA cargo can survive proteolytic digestion and that intestinal epithelial cells take up the exosomes and deliver them to the nucleus. Research on human adults consuming cow milk has shown that major bovine milk miRNAs are found in the circulation postprandi- ally, further suggesting that exosomes can resist conditions in the gastrointestinal tract and be delivered to the systemic circulation. Thus, it is possible that milk miRNAs may transfer genetic material to the infant and thereby affect gene transcription and regulation of cellular events in several tissues.
Introduction
Human milk provides many benefits to the breastfed infant resulting in significantly better short- and long-term outcomes as compared to formula-fed infants.These benefits are likely achieved by a well-balanced supply of nutrients and a wide variety of bioactive components in breast milk. These components include long-chain fatty acids (e.g., DHA), complex oligosaccharides, bioactive proteins (e.g., immunoglobulins, lactoferrin, and osteopontin), nucleotides, and lutein. By various mechanisms that have been extensively studied, they protect the infant against infections and stimulate brain development and visual function.
A novel and very fundamental mechanism to potentially affect a multitude of cells and functions in the breastfed infant is the transfer of microRNAs (miR- NAs) via human milk. Such miRNAs may affect gene transcription in the small intestine and possibly other organs and therefore have the capacity to regulate many physiological functions (Fig. 1). The miRNAs are packaged in particles, exosomes, which were recently found and characterized in breast milk [1].
MicroRNAs
The finding of RNAs of very small size was initially believed to be “metabolic by-products,” but it was soon found that this group of miRNAs, which only contain 4–20 nucleotides, exerts important regulatory roles in a large variety of cells. They are formed very specifically and their biogenesis involves 3 steps: (1) miRNA genes are transcribed by RNA polymerase II or III into primary miRNA transcripts with (2) subsequent cleavage by the microprocessor complex Drosha-DGCR8 in the nucleus, resulting in a precursor hairpin (pre-miRNA), which is exported out of the nucleus for (3) final processing into mature miRNAs by the enzyme RNase Dicer [2]. miRNAs bind through partial sequence homology to the 3′-untranslated region of target mRNAs and cause either translational block or mRNA degradation. While most of the early research on miRNAs focused on their significance in various forms of cancer and biomarker discovery, it was subsequently found that they are also present in various foods, such as milk, meat, and plants.
Exosomes in Milk
Exosomes are small extracellular vesicles about 30–100nm in size and are produced by a variety of cells including macrophages, lymphocytes, dendritic cells, and epithelial and tumor cells [3]. They are found in physiological fluids such as saliva, plasma, and urine [4]. It is well known that exosomes are important in cell-cell signaling, but their physiological significance in vivo is less known. Early studies suggested promising roles in immunotherapy and cancer therapy, but this is a rapidly advancing field and many clinical trials are ongoing.
The presence of exosomes in breast milk was first described by Admyre et al. [1], who also showed that isolated milk exosomes could affect immune respons- es of peripheral blood mononuclear cells (PBMCs) and T-regulatory cells. Exo- somes contain specific marker proteins, such as MHC classes I and II, CD63, CD81, and CD86 [1], which may be involved in cell recognition, but, more importantly, miRNAs have the capacity to regulate transcriptional activity. It was subsequently shown that breast milk exosomes contain RNA, and that this RNA is short (20–35 nucleotides) and does not contain ribosomal RNA [5]. Kosaka et al. [6] studied miRNA expression in breast milk by microarray analysis and found large numbers of miRNAs (281 of 723 human miRNAs known at that time) and in particular high levels of immune-related miRNAs during the first 6 months of lactation. Among the major miRNAs detected were miR-181a and miR-181b, which are regulators of B-cell differentiation and CD4+ T-cell selection; miR-155, a regulator of T- and B-cell maturation and immune responses; the miR-17–92 cluster, which is a ubiquitous regulator of B-cell, T-cell, and monocyte development, and miR-125b, a negative regulator of TNF-α production, activation, and sensitivity. They found that miRNA expression in breast milk samples from the same mother did not vary greatly with time after birth. Treatment of breast milk with RNAse had little or no effect on the miRNAs, nor had freeze-thaw cycles or exposure to acidic pH (pH 1), suggesting that they may survive in the gastrointestinal tract. It was proposed that their presence in exosomes may shield them from the effects of low pH and digestive enzymes.
Further deep sequencing of breast milk exosomes revealed 602 unique miRNAs, that they are in a narrow size range (20–24 nucleotides), and that many of them are involved in immune function [7]. Although they only analyzed milk from 4 mothers, their results suggested low intersubject variability. They further exposed raw milk with added synthetic exogenous (not milk-related) miRNAs to various harsh conditions (prolonged room temperature, freeze-thawing cycles, RNase incubation, and high temperature exposure) and found that the exogenous miRNAs were rapidly degraded, but not the endogenous milk miRNAs. This strengthens the hypothesis that their presence in exosomes in milk protects them from digestion.
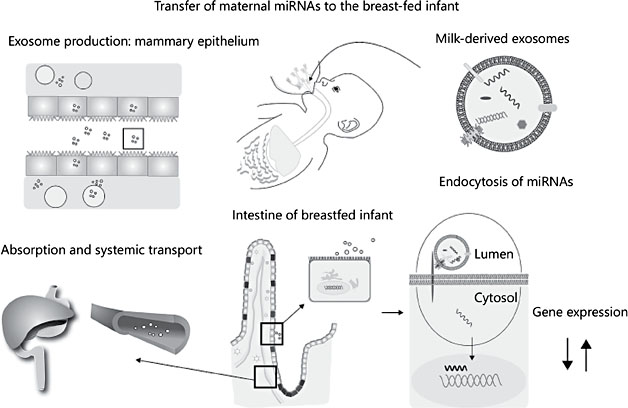
Fig. 1. Synthesis, transport, and functions of human milk-derived miRNAs.
Origin of Milk MicroRNAs and Variation during Lactation
To explore the origin of human milk miRNAs, Alsaweed et al. [8] used next- generation sequencing to analyze miRNAs in human milk cells and fat, and compared them to miRNAs in maternal PBMCs and plasma. They found a strong association in miRNA profiles between human milk cells and fat, but miRNAs in PBMCs and plasma were distinctly different. Since most cells in mature breast milk are epithelial cells, they suggested that the milk miRNAs primarily originate from the mammary epithelium with a smaller contribution from the maternal circulation [8]. They also analyzed infant formulas and found them to contain very few human miRNAs and at very low abundance.
The variation in miRNA content and composition during the lactation period has been characterized [9]. Total miRNA concentration was similar at 3 lactation stages (2, 4, and 6 months) as were the top 20 known miRNAs and the number and expression of known miRNAs in human milk fractions. However, about one-third of the known miRNAs were differentially expressed during the first 6 months of lactation, with more pronounced upregulation at 4 months. The authors concluded that although the total miRNA quantity delivered to infants does not change during the first 6 months of lactation, the composition is altered, particularly at 4 months, which may reflect remodeling of the mam- mary gland in response to partial weaning [9]. We also compared total miRNAs during lactation and found little variation, but we did find that several of the top 15 milk miRNAs showed considerable variation [10]. This may be due to the limited number of subjects/samples analyzed in these studies. Interestingly, 11 out of the 15 top miRNAs found in our study of US women were also found in the study of Australian women [9]. Similarly, all top 10 miRNAs identified by Zhou et al. [7] in milk from Chinese women were found among the top 30 miRNAs analyzed in our study, indicating that the major miRNA species in human milk are not much affected by maternal ethnicity.
The composition of preterm human milk is known to be different than that of term milk, and in a recent study metabolism-related miRNAs were found to be affected by premature delivery [11]. There were 113 miRNAs with significant expression differences between preterm and term milk lipids, most of which have been described in earlier studies. The authors selected the 15 miRNAs with the most significant differences for functional analysis and found that the most prominent mRNA targets are involved in cellular nitrogen metabolism, biosynthesis, catabolism, symbiosis, and viral processing. The pathway with the most significant enrichment in miRNA targets in preterm milk was glycosphingolipid biosynthesis [11], which is interesting as glycosphingolipids are involved in neurodevelopment [12]. It was also found that miRNAs in term colostrum were similar to those in term mature milk, and that both differed substantially from preterm milk. The authors suggested that premature delivery in itself may affect the miRNA composition in milk as 21 of 26 miRNAs were significantly related to premature delivery, and 6 of them correlated with the delivery method (cesarean section or vaginal delivery). The possibility of miRNA production in the cell nucleus being affected by the rapid shift in maternal hormones during the late preterm and early postpartum period was also discussed.
Effect of Digestion on Milk Exosomes
Whereas some previous in vitro studies indicated that milk exosomes and their miRNA cargo can resist harsh conditions like acid, boiling, and RNase treatment, these conditions are not the same as those in the recipient infant being breastfed. We have exposed breast milk to a gastric pH commensurate with an infant stomach pH (4.0) and incubated with pepsin for 20 min, then adjusted the pH to 7.0, similar to what would happen with the secretion of pancreatic fluid, followed by incubation for a further 30 min with pancreatin [10]. We found that normalized reads of the top 15 miRNAs isolated from human milk exosomes before and after in vitro digestion were very similar, and that they had similar overall abundance distribution.
Bovine milk was also found to contain microvesicles carrying miRNAs [13], among them miR-101, miR-125b, miR-150, miR-223, miR-24-1, and miR-93. To explore whether humans can absorb biologically meaningful amounts of miRNAs from commercial cow milk, Baier et al. [14] gave varying volumes of milk to 5 adult volunteers and analyzed 2 major milk miRNAs (miR-29b and miR-200c) in PBMCs. Plasma time curves showed an increase in miR-29b, which was highest at 4 h and had returned to baseline by 9 h. The AUC (area under the curve) showed a linear dose-response to the volume of milk ingested, suggesting that milk-based miRNAs indeed can survive digestion and be ab- sorbed and thus transferred to various cells and tissues. Studies in human Caco- 2 cells and rat IEC-6 cells suggest that the intestinal uptake of exosomes is medi- ated by endocytosis [15].
The bioavailability of dietary miRNAs is controversial. The study by Baier et al. [14] strongly suggests that part of milk-borne miRNAs is absorbed, which may be explained by their presence in milk exosomes. As described above, the structure of the milk exosome may at least in part protect the miRNAs from the conditions in the gut. When 2 miRNAs from broccoli were analyzed in plasma from subjects participating in a broccoli feeding study, they were below the detection limit [14]. It is thus possible that the miRNAs in plant-based diets are more vulnerable to digestion, or that the human intestine is less capable of absorbing plant exosomes. Experiments in various knockout and knock-in mouse models ad- dressing this research question are less convincing [16]. When miR-30b was overexpressed 134 times in transgenic mice, the authors found no effect of the increased level of this miRNA in milk on pup tissue levels [17]. As pointed out by Melnik et al. [16], they did not assess whether the additional miRNA in the milk was present in exosomes, which affects its stability. Since the extra miR-30b was substantially lower in stomach contents of the mice than in the milk, it is possible that the overexpressed miRNA was in another milk compartment and more vulnerable to proteolysis and possibly also less bioavailable. Knockout mouse pups lacking miR-375 and miR-200c/141 and nursing wild-type mothers were used by Title et al. [18] to assess transfer of these miRNAs to the systemic circulation. They found a very small increase in plasma levels of these miRNAs and concluded that milk miRNAs do not play a genetic regulatory role in newborn mammals. However, these miRNAs are involved in control of endocytotic events and epithelial cell function, and hence mechanisms for exosome/miRNA uptake in the pups may have been impaired [16], making this a less convincing model.
Cellular Uptake of Exosomes/MicroRNAs
Studies have shown intestinal uptake of undigested milk exosomes in pigs and rats [19, 20]. We exposed human intestinal epithelial crypt-like cells to exosomes isolated from breast milk that was undigested or subjected to in vitro digestion as described above [10]. The digestive fate is important for major nutrients like proteins and lipids, but also for extracellular vesicles, such as human milk exosomes. Demonstration of intestinal cell uptake after gastric/pancreatic digestion would provide support for the notion that exosomes are vehicles for transfer of genetic material from the mother’s milk to her offspring. We found that the protein profiles of the exosomes exposed to digestion at both pH 4 and pH 2 were similar to those of undigested exosomes. Since we have previously shown that purified lactoferrin from human milk is degraded at pH 2 [21] and lactoferrin as a component of the exosome protein is not, it is possible that the partial compartmentation of lactoferrin into exosomes protects it from proteolysis and facilitates its uptake into the enterocyte. Using fluorescent dyes and confocal microscopy, we were able to show that the cells could readily take up exosomes from both untreated and in vitro digested breast milk at 0.5 and 2 h. At both time points, about 10% of the internalized exosomes localized to the nucleus, suggesting novel mechanisms for nuclear gene regulation conferred by human milk exosomes. Although speculative, the advantages of the delivery of gene expression regulators via breastfeeding is apparent: (1) exosomes provide a boundary that protects molecules from being attacked by low pH and a high enzymatic activity environment; (2) efficient batch recognition and internalization by the intestinal epithelium; and (3) bolus delivery for higher local concentration and therefore efficacy of action.
Functions of Breast Milk MicroRNAs
We ranked the relative abundance of all detected miRNAs in our study [10] and found 630 miRNAs of which 288 were more abundant. We noted that miR-22- 3p is consistently (from early to late lactation, undigested vs. in vitro digested) detected as the most abundant miRNA species in our study, accounting for∼20% of all top 296 miRNA read counts. miR-22-3p is an immune-related miRNA abundant in liver, targeting transcription factor 7, an important effector molecule in the Wnt pathway, which increases expression of enzymes in the liver gluconeogenic pathway, and therefore is a therapeutic target to treat insulin resistance and type 2 diabetes [22]. Its identification in human milk exosomes expands its established role of modulating carbohydrate metabolism to a potential new domain of benefits in postnatal developmental programming.
We used TargetScan to obtain target genes for the 5 most abundant miRNAs in our expression profile data. hsa-miR-30d-5p has a small number of 21 targets, whereas the other 4, hsa-miR-22-3p, hsa-miR-148a-3p, hsa-141-3p, and hsa-miR-181a-5p, all have more than 600 predicted transcripts with conserved target sites. There are 406 genes that are targets for more than 1 of these latter 4 miRNAs, and transcription regulation-related molecular functions are the most highly enriched, showing that miRNAs transferred from the mother can influence production of functional proteins that regulate the infants’ DNA decoding to RNA, which is also reflected by the highly enriched transcription- related gene ontology biological process terms. Synapse localization is also strongly enriched among these genes. Synapse formation, stabilization, and plasticity are key features of neuronal development [23], and many miRNAs have been shown to be involved in synapse plasticity [24]. We therefore surveyed miRNAs that have positive impact on mammalian synapse development, and half are present in the top 288 group of human milk exosome miRNAs. The presence of these miRNAs suggests that brain development in early life may benefit from these functions.
We then surveyed the miRNAs identified in our study for the previously reported 59 immune-related pre-miRNAs [7] and identified 57 of them, including 50 in the top 288 miRNA group [10]. We also curated a list of 86 immune-related miRNAs based on the literature, and this covered 29 of the 59 miRNAs previously identified [7]. Among the 86 immune-related miRNAs, 65 (75.6%) are present in the top 288 group reported in our study. Discovering a large number of these additional immune-related miRNAs in human milk exosomes, including miR-29a-3p, miR-29b-3p, miR-22-3p, miR-181c-5p, miR-181a-5p, miR- 181a-3p, miR-16-5p, miR-26a-5p, and miR-34a-5p, reinforces that human milk exosomes are a rich source of immune-related miRNAs.
Involvement of milk miRNAs in brain development was also suggested by Carney et al. [11] who found that the pathways with the most significant enrichment in miRNA targets from preterm milk are biosynthesis of glycosphingolipids and cell membrane function. Effects of preterm delivery on miRNAs regulating genes involved in the glycolytic pathway and glucose metabolism, as well as obesity-related genes, were also found, possibly indicating alterations in growth and metabolism of preterm infants [11]. It should also be emphasized that miRNAs are likely involved in mammary signaling during lactation, as distinct differences in many miRNAs were found when transitioning from lactogenesis to galactopoiesis and involution in dairy cows [25]. We also examined miRNAs from mothers delivering preterm infants and found that they also survive in vitro digestion and are taken up by human intestinal cells in culture [26]. We identified 330 miRNAs as preterm milk exosome miRNAs and the most abundant of these are similar to those found in term milk. Twenty-one low abundance miRNAs are specifically expressed in preterm milk exosomes compared to early term milk, possibly suggesting specific functions in the preterm infant.
Human milk cells also contain miRNAs, which have been extensively characterized [27]. The most highly expressed miRNAs were found to be key regulators of milk components and may therefore be involved in the biosynthetic pathways in the mammary gland. The authors also found miRNAs targeting genes involved in body fluid balance, thirst, appetite, immune responses, and development, possibly affecting the recipient infant and complementing the miRNAs provided by exosomes.
Finally, miRNAs are found in milk fat, whey, exosomes, and cells, and their composition varies among these compartments [9, 28, 29]. Interestingly, a maternal high-fat diet was found to affect miRNAs in milk fat globules [30], suggesting that effects of the diet of the mother should be studied in more detail. Further investigations of the composition, digestibility, and cellular uptake/ transfer, and effects of miRNAs in milk on different target cells should increase our understanding of their biological functions.
Conclusions
Human milk provides a rich source of miRNAs contained in exosomes and cells. The major miRNAs appear to be conserved among women and may therefore have biological significance for breastfed infants. These packaged miRNAs seem to survive digestion and are taken up by small intestinal cells, where they either can affect gene transcription locally or be transported by the systemic circulation to other target organs. Genes targeted by human milk miRNAs affect many pathways, in particular carbohydrate and energy metabolism, immune function, and brain development. Thus, breast milk may transfer genetic material to the infant and thereby affect gene transcription and regulation of cellular events in several tissues, possibly resulting in improved short- and long-term outcomes.
References
-
1 Admyre C, Johansson SM, Qazi KR, et al: Exosomes with immune modulatory features are present in human breast milk. J Immunol 2007;179:1969–1978.
-
2 Winter J, Jung S, Keller S, et al: Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat Cell Biol 2009;11: 228–234.
-
3 Thery C, Zitvogel L, Amigorena S: Exosomes: composition, biogenesis and function. Nat Immunol Rev 2002;2:569–579.
-
4 Théry C, Amigorena S, Raposo G, Clayton A: Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol 2006;chapter 3:unit 3.22.
-
5 Lässer C, Alikhani VS, Ekström K, et al: Human saliva, plasma and breast milk exosomes contain RNA: uptake by macrophages. J Transl Med 2011;9:9.
-
6 Kosaka N, Izumi H, Sekine K, Ochiya T: MicroRNA as a new immune-regulatory agent in breast milk. Silence 2010;1:7.
-
7 Zhou Q, Li M, Wang X, et al: Immune-related microRNAs are abundant in breast milk exosomes. Int J Biol Sci 2012;8:118–123.
-
8 Alsaweed M, Lai CT, Hartmann PE, et al: Human milk miRNAs primarily originate from the mammary gland resulting in unique miRNA profiles of fractionated milk. Sci Rep 2016;6:20680.
-
9 Alsaweed M, Lai CT, Hartmann PE, et al: Human milk cells and lipids conserve numerous known and novel miRNAs, some of which are differentially expressed during lactation. PLoS One 2016;11:e0152610.
-
10 Liao Y, Du X, Li J, Lönnerdal B: Human milk exosomes and their microRNAs survive digestion in vitro and are taken up by human intestinal cells. Mol Nutr Food Res 2017;61(11).
-
11 Carney MC, Tarasiuk A, DiAngelo SL, et al: Metabolism-related microRNAs in maternal breast milk are influenced by premature delivery. Pediatr Res 2017;82:226–236.
-
12 Lingwood CA: Glycosphingolipid functions. Cold Spring Harb Perspect Biol 2011; 3:a004788.
-
13 Hata T, Murakami K, Nakatani H, et al: Isolation of bovine milk-derived microvesicles carrying mRNAs and microRNAs. Biochem Biophys Res Commun 2010;396:528–533.
-
14 Baier SR, Nguyen C, Xie F, et al: MicroRNAs are absorbed in biologically meaningful amounts from nutritionally relevant doses of cow milk and affect gene expression in peripheral blood mononuclear cells, HEK-293 kidney cell cultures, and mouse livers. J Nutr 2014;144:1495–1500.
-
15 Wolf T, Baier SR, Zempleni J: The intestinal transport of bovine milk exosomes is mediated by endocytosis in human colon carcinoma Caco-2 cells and rat small intestinal IEC-6 cells. J Nutr 2015;145:2201–2206.
-
16 Melnik BC, Kakulas F, Geddes DT, et al: Milk miRNAs: simple nutrients or systemic functional regulators? Nutr Metab (Lond) 2016; 13:42.
-
17 Laubier J, Castille J, Le Guillou S, Le Provost F: No effect of an elevated miR-30b level in mouse milk on its level in pup tissues. RNA Biol 2015;12:26–29.
-
18 Title AC, Denzler R, Stoffel M: Uptake and function studies of maternal milk-derived microRNAs. J Biol Chem 2015;290:23680– 23691.
-
19 Chen T, Xie MY, Sun JJ, et al: Porcine milk derived exosomes promote proliferation of intestinal epithelial cells. Sci Rep 2016;6: 33862.
-
20 Hock A, Miyake H, Li B, et al: Breast milk- derived exosomes promote intestinal epithelial cell growth. J Pediatr Surg 2017;52:755– 759.
-
21 Lönnerdal B, Jiang R, Du X: Bovine lactoferrin can be taken up by the human intestinal lactoferrin receptor and exert bioactivities. J Pediatr Gastroenterol Nutr 2011;53:606–614.
-
22 Kaur K, Vig S, Srivastava R, et al: Elevated he- patic miR-22-3p expression impairs gluconeogenesis by silencing the Wnt-responsive transcription factor Tcf7. Diabetes 2015;64: 3659–3669.
-
23 Cao DD, Li L, Chan WY: MicroRNAs: key regulators in the central nervous system and their implication in neurological diseases. Int J Mol Sci 2016;17:E482.
-
24 Aksoy-Aksel A, Zampa F, Schratt G: MicroR- NAs and synaptic plasticity – a mutual relationship. Philos Trans R Soc Lond B Biol Sci 2014;369:20130515.
-
25 Do DN, Li R, Dudemaine PL, et al: MicroR- NA roles in signaling during lactation: an insight from differential expression, time course and pathway analyses of deep sequence data. Sci Rep 2017;7:44605.
-
26 Kahn S, Liao Y, Du X, et al: Exosomal microRNAs in milk from mothers delivering preterm infants survive in vitro digestion and are taken up by human intestinal cells. Mol Nutr Food Res 2018;62(11):e1701050.
-
27 Alsaweed M, Hartmann PE, Geddes DT, Kakulas F: MicroRNAs in breastmilk and the lactating breast: potential immunoprotectors and developmental regulators for the infant and the mother. Int J Environ Res Public Health 2015;12:13981–14020.
-
28 Munch EM, Harris RA, Mohammad M, et al: Transcriptome profiling of microRNA by Next-Gen deep sequencing reveals known and novel miRNA species in the lipid fraction of human breast milk. PLoS One 2013; 8:e50564.
-
29 Alsaweed M, Hepworth AR, Lefèvre C, et al: Human milk microRNA and total RNA differ depending on milk fractionation. J Cell Bio- chem 2015;116:2397–2407.
