Human Milk Banking
Key Messages
- Donor milk from human milk banks is essential for premature and sick infants.
- Milk banks strictly follow guidelines for the storage, processing, and handling of human milk to ensure the safety of donor milk.
- Pasteurized donor milk retains many of the beneficial health effects of raw human milk.
Key Words
Human milk banking · Donor milk · Milk processing · Pasteurization · Preterm infants · Sick infants
Abstract
Human milk banks play an essential role by providing human milk to infants who would otherwise not be able to receive human milk. The largest group of recipients are premature infants who derive very substantial benefits from it. Human milk protects premature infants from necrotizing enterocolitis and from sepsis, two devastating medical conditions. Milk banks collect, screen, store, process, and distribute human milk. Donating women usually nurse their own infants and have a milk supply that exceeds their own infants’ needs. Donor women are carefully selected and are screened for HIV-1, HIV-2, human T-cell leukemia virus 1 and 2, hepatitis B, hepatitis C, and syphilis. In the milk bank, handling, storing, processing, pooling, and bacterial screening follow standardized algorithms. Heat treatment of human milk diminishes anti-infective properties, cellular components, growth factors, and nutrients. However, the beneficial effects of donor milk remain significant and donor milk is still highly preferable in comparison to formula.
Introduction
It is probably not widely appreciated that human milk banking is an absolute necessity if all infants are to enjoy the benefits of human milk. This is so because a substantial number of infants, especially premature infants, are unable to receive adequate amounts of their mothers’ milk for a variety of reasons. Were it not for milk banks, these infants could not be fed human milk and would suffer the consequences. Premature infants derive very important protections from human milk.
Unfortunately, there are circumstances where milk from the infant’s own mother is not available. Milk donated by other women (donor milk) must then fill the gap. Premature infants constitute the largest and most important group of infants where milk from other women is needed because their own mothers’ milk is not available or is not available in sufficient quantity. Human milk banks collect, screen, pasteurize, and distribute donated breast milk to hospitals or outpatient recipients [1] . Usually the collection, storage, and processing in a human milk bank follows established guidelines. Milk banks are by far the most important providers of donor milk, the fact not withstanding that other venues of milk donation are also used.
History
The first human milk bank was founded in 1909 in Vienna, Austria. Wet nursing was widely practiced in Europe during the 19th century in order to provide human milk for infants whose mothers were unable to provide milk for their infants. However, wet nurses were not always available or, when available, pursued unhealthy lifestyles or carried infections that could be transmitted through milk. An alternative to wet nursing was found in human milk banking.
Shortly after Vienna, the first milk bank opened in the United States in the Boston Floating Hospital and many others followed all over the world. In the 1960s, efforts in human milk banking faded due to advances in neonatal medical care and infant nutrition, mainly the development of high-quality infant formulas. In the 1980s, the new infectious disease, HIV, arrived. As it is transmissible through breast milk, this led to the closing of many milk banks. Once disease transmission via human milk was recognized as a health hazard, serological testing of the mother became necessary. The added financial burden drove some milk banks out of business. Appropriate screening of donating mothers as well as adherence to standards of procedure have reversed that trend since the early 2000s.
Milk banking activity varies greatly between different parts of the world due to a variety of reasons: sometimes the reasons have to do with economics and funding, and sometimes they are linked to religious and cultural factors. Globally, there is an increase of interest in milk banking all over the world. Currently there is a move to open many milk banks in India and other Asian countries such as Vietnam, China, and Japan. The increase of interest goes along with the recommendations of large pediatric societies, such as ABM, ESPHGAN, and AAP, to promote human milk feeding in premature infants [2–4]. All guidelines say that the mother’s own milk is the first choice for an infant. However, if the mother’s own milk is not available, donor milk is the recommended alternative. A further important recommendation is that donor human milk should be provided by an established human milk bank that follows standard safety guidelines.
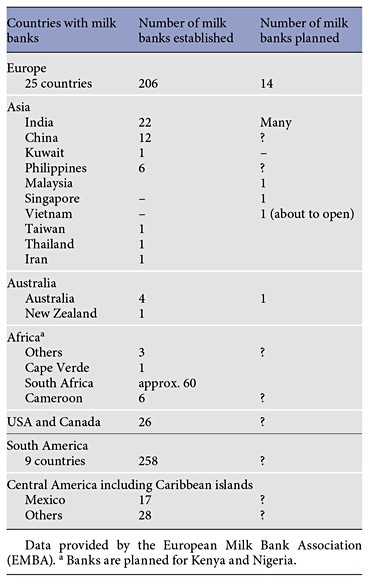
Table 1 gives an overview about the established and planned milk banks all over the world.
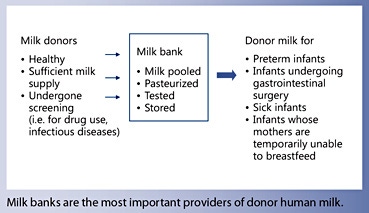
Why Is Human Milk Being Banked?
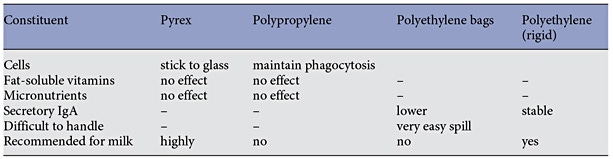
The main function of milk banks is to serve as repositories of donated milk so it is available when needed. Milk banks receive milk from donors, process it, and store it until used. Most commonly milk from multiple donors is pooled, although some banks pool milk only of individual donors (single-donor banks). Usually, milk provided by milk banks has undergone pasteurization. Once pasteurized, milk is placed in small (100–150 mL) containers and is stored frozen for up to 1 year depending on local guidelines. In the US, milk banks charge a processing fee to cover the costs of collecting and handling of the milk. The type of container affects the stability of the constituents of human milk, while colostrum was reported to remain stable when refrigerated for 24 h in any container (Table 2) [5].
Who Are the Milk Donors?
Most milk is donated by women, who have nursed their own infant for some time and realize that their milk supply is large enough to allow them to donate milk while still satisfying their own infant’s needs. A study from France [6] showed that the typical donating mother was of average childbearing age with strong support at home. Almost half did not work outside of the home, and a large number were from the health and social services fields. Reasons for donation were largely altruistic, and a general optimistic attitude prevailed within the mothers.
To be eligible as milk donors, women must not be using recreational or other drugs, and their physician as well as the infant’s physician must agree that milk donation is in order. The milk bank will obtain a health history and obtain blood for testing. Usually, the donating mother is screened for HIV-1, HIV-2, human T-cell leukemia virus 1 and 2, hepatitis B, hepatitis C, and syphilis [7]. If all requirements are met, the donor is provided a supply of milk containers and receives instruction as to the appropriate means of milk expression. The donor obtains milk by mechanical pump or manual expression and stores it in the freezer compartment of their home refrigerator before delivery to the milk bank. The milk is transported to the milk bank either by the mother herself or by a transport service provided by the milk bank. It is important that the cooling chain is never interrupted; therefore, special special cooling bags or cooling boxes have to be used during transportation from home to the milk bank.
Milk donation is an act of unselfishness. In most countries donors receive no compensation, but in some countries donors receive modest monetary compensation for actual costs incurred. It represents mostly a token of appreciation.
How Is Milk Handled by the Bank?
Milk banks generally follow standardized procedures for the collection and handling of donated milk [7]. Donors are instructed by the milk bank about recommended breast cleaning and breast pumping procedures. The bank provides containers for milk. Pooling of milk from several pumpings is often performed. Each container must carry the name, date, and time of expression. The milk remains in the freezer until it is delivered to the bank. Figure 1 shows the human milk banking process.
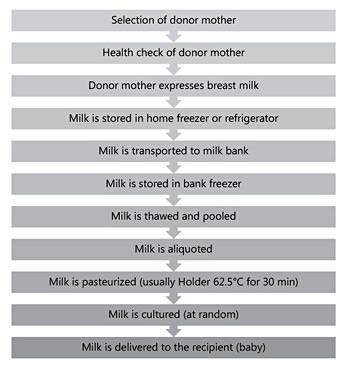
At the bank, milk is stored at –20 ° C. On the day before processing, the donated milk is placed in a refrigerator for overnight thawing. On the day of pasteurization, the milk from 3–5 donors is pooled. Pooling serves the purpose of distributing nutrients, such as protein and fat, as well as foreign substances evenly. After pooling, the milk is placed in individual 100 mL bottles. Pasteurization is carried out in a water bath at 62.5 ° C for 30 min followed by rapid cooling. Milk bottles are then stored at –20 ° C until use of the milk. This method (Holder pasteurization) is widely felt to represent a good compromise between microbiological safety and nutritional/biological quality of donor milk [8] Nevertheless, methods that lead to less nutrient loss and, perhaps, are less time consuming would be desirable and are being sought [9].
- High-temperature short-time pasteurization at 72°C for 5–15 s is such a method. It reaches a better compromise between microbiological safety and nutritional and biological quality of donor milk [10–13]. The method is not yet used routinely due to lack of suitable instrumentation.
- The combination of ultrasound and heat (thermoultrasonic treatment) is an emerging technique that allows milk to retain more of its bioactive components compared with thermal pasteurization [14]. However, the current experimental system is limited to small volumes and needs to be scaled up.
- High-pressure processing (HPP) shows promise as an alternative to pasteurization. Total immunoglobulin A immunoreactivity and lysozyme activity are significantly higher after HPP compared with pasteurization [15]. Besides, HPP is faster and probably more convenient than Holder pasteurization. It seems a promising technology, but further investigation is necessary before it can be used routinely.
- Finally, there is Ohmic heating, a new technology under investigation. Ohmic treatment is a thermal processing method wherein the food material, which serves as an electric resistor, is heated by passing an electric current through it, which leads to rapid and uniform heating. Like thermal processing, Ohmic heating inactivates microorganisms by heat. The first experimental trials have shown no modification of the protein pattern of milk at a temperature of 72°C and only small changes at a temperature of 78°C.
In some countries, the milk is tested bacteriologically before it is pasteurized, in some countries after pasteurization, and in others the milk is tested before and after pasteurization. Some countries, such as Norway, have a tradition of feeding raw donor milk. In Norwegian milk banks, each container of milk from a donor is screened for bacteria. Milk that contains any pathogens or high counts (>100,000 colony-forming units/mL) of any other bacteria is destroyed. Milk with a low bacterial count (<10,000 colony-forming units/mL) is used for the smallest preterm babies [16]. Ronnestad et al. [17] described an incidence of late-onset sepsis in a Norwegian cohort of extremely low-birth-weight infants receiving raw breast milk or donor milk of 19.8% (80/405), which was similar to the incidence in the Vermont Oxford quality network (21.4%, in the year 2000). Therefore, it is unlikely that microbiologically screened raw milk is hazardous for preterm infants.
Who Are the Recipients of Donor Milk?
The most common recipients of donor milk are the following [18]:
- Premature infants, especially infants with a birth weight below 1,500 g, because of their high risk of infection and necrotizing enterocolitis
- Infants with gastrointestinal anomalies undergoing gastrointestinal surgery leading to short bowel syndrome
- When the mother is temporarily unable to nourish her infant completely, e.g. when the mother is ill or hospitalized
- Weaning from parenteral nutrition
- Metabolic disorders, especially amino acid disorders
- Before the mother’s own milk comes in (first few days after birth).
Premature infants are not only the largest group of recipients of donor milk, they are also those who derive by far the greatest benefits from receiving human milk. Human milk exerts strong trophic effects on the infant gut and thereby enables full enteral feedings to be reached earlier than without human milk [19]. Human milk protects premature infants strongly against necrotizing enterocolitis [19, 20] and against sepsis [21], two conditions that carry high mortalities. Some mothers object intuitively to the use of donor milk, which is why donor milk is fed only after its source has been explained to the mother and she has agreed to its use.
The reason why mothers of premature infants are often not able to provide milk at all or provide milk only in insufficient quantity is that premature delivery, by shortening pregnancy, foreshortens the period of preparatory lactogenesis. Also, the necessary mechanical milk expression is less effective in stimulating and maintaining milk production than suckling by a mature infant.
It has been suggested that the availability of donor milk could act as a disincentive to mothers to provide milk for their premature infants. There are indeed data in the literature that support this contention [22]. However, a 1- year study in 2010 involving all NICUs in Italy showed that the rate of exclusive breastfeeding at the time of discharge was significantly higher in NICUs with milk banks than in NICUs without milk banks (29.6 vs. 16.0%) [23]. This confirms anecdotal reports from other areas. It appears thus that the availability of donor milk has a positive effect on the motivation of mothers to provide milk for their infants.
Donor milk is also fed to older babies and to children with a variety of medical conditions, including severe food allergy or feeding intolerance, growth failure while on formula, intractable rotavirus enteritis, and during chemotherapy for cancer [24]. Occasionally, adopted infants receive donor milk. Furthermore, there are several case reports where donor milk was used in adults with special medical conditions, e.g. in liver transplanted patients who were IgA deficient to supply extra IgA [25] or in adult cancer patients [26].
The Composition of Donor Milk
It is widely appreciated that the composition, in particular the protein and fat content, of individual expressions of human milk varies greatly. This has led to the promotion of bedside human milk analyzers and procedures for nutrient fortification of individual milk samples. However, with donor milk the variability of composition is greatly reduced due to pooling. Milk from multiple pumpings is usually pooled by the donor mother before delivery to the bank. Pooling of milk from multiple donors is then performed by the milk bank, with the result that the protein and fat content of pooled milk is quite stable and predictable. Michaelsen et al. [27] reported that fat and protein concentration vary widely from sample to sample but that variability decreases sharply with pooling of samples from multiple donors. At the Mother’s Milk Bank of Iowa, the nutrient content of 37 milk pools collected over a period of 2 years (2003–2005) was analyzed. The (true) protein concentration averaged 8.22 g/L with an SD of 0.59 g/L, and the fat content averaged 39.0 g/L with an SD of 3.51 g/L. The variability of composition is thus far lower than that between individual samples [28]. Low variability is of particular advantage in the case of premature infants because variability of the protein content is often the source of concerns about inordinately high intakes of protein. Low variability of composition of donor milk has the advantage that the infant’s nutrient intake varies little from feeding to feeding and is essentially always known, whereas with the mother’s own milk the nutrient content may vary greatly from feeding to feeding.
Is Donor Milk as Good as Mother’s Milk?
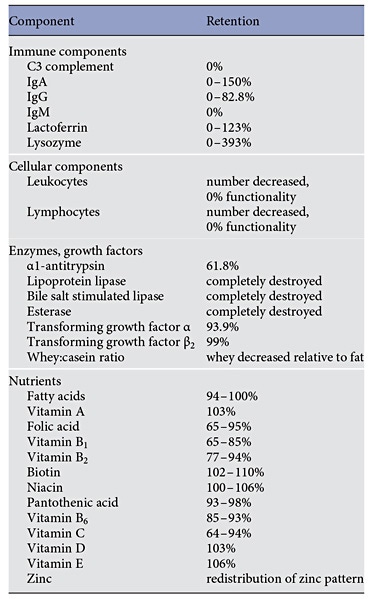
The fact that, with some exceptions, donor milk undergoes pasteurization has led to concerns that some or all of the protective effects of human milk may be lost. Studies assessing milk components before and after pasteurization have documented that indeed several important components of human milk are reduced in concentration or are eliminated altogether, as summarized in Table 3. Heat treatment affects anti-infective and cellular components, growth factors, and some nutrients, depending on the heat and duration of exposure. Enzymes are most heat-sensitive while immune components are compromised but not completely destroyed.
Processing of human milk also affects unsaturated fatty acids [29] and damages the membrane of milk fat globules [30]. Human milk contains stem cells with multilineage properties and variable expression of pluripotency genes normally found in human embryonic stem cells [31]. It is likely that these stem cells are destroyed during heat treatment. On the other hand, some important protective components such as the oligosaccharides are essentially resistant to the effects of heat.
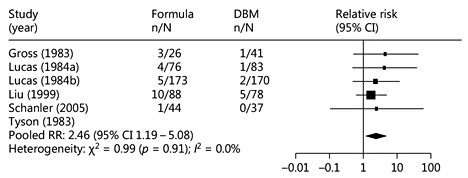
Given these effects of high-temperature processing, it would be expected that the protective effects of human milk might be diminished but not abolished altogether. That is exactly what the literature shows. In 5 trials comparing formula with donor milk with regard to the incidence of necrotizing enterocolitis, the risk of necrotizing enterocolitis was nonsignificantly diminished in each trial. However, collectively, the 5 trials showed a significant protective effect of donor milk compared to formula (Fig. 2) [32]
A direct comparison of fresh against pasteurized human milk performed by Narayanan et al. [33] showed a somewhat reduced protective effect against infection (14.3 vs. 10.5% infection) which was still much stronger than the effect of formula (33.3% infection). It is thus evident that the beneficial effects of pasteurized human milk are diminished vis-à-vis fresh milk but that enough of the protective effects remain to render donor milk the feeding of choice for premature infants in the absence of any or sufficient maternal breastmilk.
Is Donor Milk Safe?
Because of the potential for transmission of disease pathogens, sometimes there are concerns about this possibility. With current donor screening and pasteurization of donor milk, the possibility of disease transmission is infinitesimally small. In fact, there is not a single case of documented disease transmission through banked donor milk in recent decades. Whether that can also be said about informal milk exchanges is not known.
Is Donor Milk Cost-Effective?
Because milk banks charge a processing fee ($6–7/100 mL donor milk), it has been asked whether the benefits accruing to the infants justify the expense. Although it is inappropriate to ask this question in relation to deadly conditions (necrotizing enterocolitis, sepsis), fortunately several studies have documented that the use of donor milk is cost-effective [34, 35]. Thus, the use of donor milk not only saves lives, it also saves the hospital money. It was reported that sometimes mothers buy breast milk for their premature or sick infants via the internet, social networks [36], from friends, from private providers, or in other informal sharing arrangements. In those cases, donor screening, quality control of the human milk, and shipping standards are missing. This behavior is risky and is not recommended.
All donor milk must be fortified with nutrients before it is fed to premature infants. In this regard, donor milk does not differ from the mother’s own milk. Most human milk fortifiers contain as protein source various fractions or derivatives of cow milk. One fortifier provides protein from human milk and is claimed to protect better against necrotizing enterocolitis than fortifiers containing bovine milk proteins, although evidence for that effect is lacking [37]. The lack of benefit, therefore, argues against use of the high-cost human milk-based fortifier.
Conclusion
Milk banks serve a vital function by providing human milk for premature infants who, for a variety of reasons, would otherwise not have access to human milk. As human milk confers major protective effects to premature infants, the availability of human milk is an important quality of care issue. The use of donor milk is widely endorsed [4, 38, 39].
Acknowledgements
We thank Gillian Weaver, head of the European milk banking association EMBA, for her valuable input.
Disclosure Statement
The authors declare that no financial or other conflict of interest exists in relation to the contents of this article. The writing of this article was supported by Nestlé Nutrition Institute.
References
- Arnold LDW: Human Milk in the NICU: Policy into Practice. Ontario, Jones and Bartlett Publishers, 2010.
- Arslanoglu S, et al; ESPGHAN Committee on Nutrition: Donor human milk for preterm infants: current evidence and research directions. J Pediatr Gastroenterol Nutr 2013; 57: 535–542.
- The Academy of Breastfeeding Medicine: ABM Clinical Protocol #10: Breastfeeding the late preterm infant (34 0/7 to 36 6/7 weeks gestation) (first revision June 2011). Breastfeed Med 2011; 6: 151–156.
- Eidelman AI, Schanler RJ: Breastfeeding and the use of human milk. Pediatrics 2012; 129: e827–e841.
- Goldblum RM, et al: Human milk banking. II. Relative stability of immunologic factors in stored colostrum. Acta Paediatr Scand 1982; 71: 143–144.
- Azema E, Callahan S: Breast milk donors in France: a portrait of the typical donor and the utility of milk banking in the French breastfeeding context. J Hum Lact 2003; 19: 199–202.
- Human Milk Banking Association of North America (HMBANA): Guidelines for the Establishment and Operation of a Donor Human Milk Bank, 2015.
- Moro GE, Arslanoglu S: Heat treatment of human milk. J Pediatr Gastroenterol Nutr 2012; 54: 165–166.
- Moro GE: V. Processing of donor human milk. J Pediatr Gastroenterol Nutr 2015; 61(suppl 1):S6–S7.
- Goldblum RM, et al: Rapid high-temperature treatment of human milk. J Pediatr 1984; 104: 380–385.
- Hamprecht K, et al: Cytomegalovirus (CMV) inactivation in breast milk: reassessment of pasteurization and freeze-thawing. Pediatr Res 2004; 56: 529–535.
- Arslanoglu S, et al: Guidelines for the establishment and operation of a donor human milk bank. J Matern Fetal Neonatal Med 2010; 23(suppl 2):1–20.
- Baro C, et al: Effect of two pasteurization methods on the protein content of human milk. Front Biosci (Elite Ed) 2011; 3: 818–829.
- Czank C, Simmer K, Hartmann PE: Simultaneous pasteurization and homogenization of human milk by combining heat and ultrasound: effect on milk quality. J Dairy Res 2010; 77: 183–189.
- Permanyer M, et al: Maintenance of breast milk Immunoglobulin A after high-pressure processing. J Dairy Sci 2010; 93: 877–883.
- Grøvslien AH, Grønn M: Donor milk banking and breastfeeding in Norway. J Hum Lact 2009; 25: 206–210.
- Ronnestad A, et al: Late-onset septicemia in a Norwegian national cohort of extremely premature infants receiving very early full human milk feeding. Pediatrics 2005; 115: e269–e276.
- Lawrence RA, Lawrence RM: Breastfeeding: A Guide for the Medical Profession, ed 7. Missouri, Elsevier, 2010.
- Schanler RJ, Shulman RJ, Lau C: Feeding strategies for premature infants: beneficial outcomes of feeding fortified human milk versus preterm formula. Pediatrics 1999; 103: 1150–1157.
- Meinzen-Derr J, et al: Role of human milk in extremely low birth weight infants’ risk of necrotizing enterocolitis or death. J Perinatol 2009; 29: 57–62.
- Patel AL, et al: Impact of early human milk on sepsis and health-care costs in very low birth weight infants. J Perinatol 2013; 33: 514– 519.
- Davanzo R, et al: Breastfeeding at NICU discharge: a multicenter Italian study. J Hum Lact 2013; 29: 374–380.
- Arslanoglu S, et al: Presence of human milk bank is associated with elevated rate of exclusive breastfeeding in VLBW infants. J Perinat Med 2013; 41: 129–131.
- Tully MR: A year of remarkable growth for donor milk banking in North America. J Hum Lact 2000; 16: 235–236.
- Merhav HJ, et al: Treatment of IgA deficiency in liver transplant recipients with human breast milk. Transpl Int 1995; 8: 327–329.
- Rough SM, et al: Qualitative analysis of cancer patients’ experiences using donated human milk. J Hum Lact 2009; 25: 211–219.
- Michaelsen KF, et al: Variation in macronutrients in human bank milk: influencing factors and implications for human milk banking. J Pediatr Gastroenterol Nutr 1990; 11: 229–239.
- Cooper AR, et al: Macronutrient content of donor human breast milk. Arch Dis Child Fetal Neonatal Ed 2013; 98:F539–F541.
- Lavine M, Clark RM: Changing patterns of free fatty acids in breast milk during storage. J Pediatr Gastroenterol Nutr 1987; 6: 769–774.
- Schmidt E: Effects of varying degrees of heat treatment on milk protein and its nutritional consequences. Acta Paediatr Scand Suppl 1982; 296: 41–43.
- Hassiotou F, Geddes DT, Hartmann PE: Cells in human milk: state of the science. J Hum Lact 2013; 29: 171–182.
- Chauhan M, Henderson G, McGuire W: Enteral feeding for very low birth weight infants: reducing the risk of necrotising enterocolitis. Arch Dis Child Fetal Neonatal Ed 2008; 93:F162–F166.
- Narayanan I, et al: A planned prospective evaluation of the anti-infective property of varying quantities of expressed human milk. Acta Paediatr Scand 1982; 71: 441–445.
- Arnold LD: The cost-effectiveness of using banked donor milk in the neonatal intensive care unit: prevention of necrotizing enterocolitis. J Hum Lact 2002; 18: 172–177.
- Carroll K, Herrmann KR: The cost of using donor human milk in the NICU to achieve exclusively human milk feeding through 32 weeks postmenstrual age. Breastfeed Med 2013; 8: 286–290.
- Gribble KD: Peer-to-peer milk donors’ and recipients’ experiences and perceptions of donor milk banks. J Obstet Gynecol Neonatal Nurs 2013; 42: 451–461.
- Sullivan S, et al: An exclusively human milkbased diet is associated with a lower rate of necrotizing enterocolitis than a diet of human milk and bovine milk-based products. J Pediatr 2010; 156: 562–567.e1.
- Arslanoglu S, et al: Donor human milk in preterm infant feeding: evidence and recommendations. J Perinat Med 2010; 38: 347–351.
- Moro GE, et al: XII. Human milk in feeding premature infants: consensus statement. J Pediatr Gastroenterol Nutr 2015; 61(suppl 1): S16–S19.