Human Milk and Clinical Outcomes in Preterm Infants
Abstract
The LOVE MOM cohort (Longitudinal Outcomes of VLBW Infants Exposed to Mothers’ Own Milk; NIH: R010009; Meier PI) enrolled 430 infants with very low birth weight (VLBW) between 2008 and 2012 to study the impact of the dose and exposure period of MOM during hospitalization in the neonatal intensive care unit (NICU) on potentially preventable complications of prematurity and their associated costs. In this prospective study, MOM and formula feedings were calculated daily (mL), medical diagnoses for NICU morbidities (necrotizing enterocolitis [NEC], late-onset sepsis [sepsis], and bronchopulmonary dysplasia [BPD]) were confirmed independently by 2 neonatologists, and propensity scoring was used to analyze covariates. Neurodevelopmental outcome was measured for a subset of 251 LOVE MOM infants at 20 months of age, corrected for prematurity (CA). Data revealed a dose-response relationship between higher amounts of MOM received during critical NICU exposure periods and a reduction in the risk of NEC, sepsis, BPD, and their costs, as well as higher cognitive index scores at 20 months CA. MOM appears to function via different mechanismsduring NICUexposureperiodstoreducetherisk of potentiallyprevent- able complications and their costs in VLBW infants. Institutions should prioritize the economic investments needed to acquire, store, and feed high-dose MOM in this population.
Introduction
Historically, human milk (HM; includes mother’s own milk [MOM] and donor HM [DHM]) was prioritized for the feeding of premature infants throughout the world [1, 2]. However, the first actual research comparison of HM and non-HM was a case-control study that evolved from the observation that retrolental fibroplasia, known today as retinopathy of prematurity, seemed to occur less frequently in premature nurseries where HM feedings were used [3]. In the ensuing decades, there was little interest in the scientific study of clinical outcomes of HM feedings in premature infants, and the primary reason to support MOM feeding in the neonatal intensive care unit (NICU) was to support maternal involvement in infant care. The state of this science was changed completely in the mid-1980s with the study by Lucas et al. [4] who randomized 926 premature infants to different feeding interventions including MOM, DHM, and preterm and term for- mulas. Known as the Lucas cohort, participating infants were studied during the initial hospitalization, infancy, childhood, and young adulthood, resulting in the most comprehensive set of clinical outcome data on the impact of MOM, DHM, and formula feeding for premature infants available at that time [4, 5]. Their primary hypothesis about the role of early nutrition in the programming of these outcomes is still relevant today [6]. Clinically, these data spearheaded changes in NICU best practices, with a new focus on encouraging mothers to provide their MOM, even though their initial intent may have been to formula feed [7].
In the past 20 years, multiple studies have reported that MOM feedings reduce the risk of potentially preventable complications of prematurity that occur during NICU hospitalization, including necrotizing enterocolitis (NEC), late-onset sepsis (sepsis), and bronchopulmonary dysplasia (BPD) [8]. These morbidities significantly increase the risk for subsequent neurodevelopmental problems and other chronic health conditions throughout childhood, as evidenced by the high rates of rehospitalization and special education needs in this population [8, 9]. Furthermore, NEC, sepsis, and BPD significantly increase the marginal costs of NICU care that are attributable both to prolonged length of the NICU hospitalization and to greater resource use when compared to infants who do not acquire these morbidities (Fig. 1) [10, 11]. Although this body of research suggested a cost-benefit to the feeding of high-dose MOM during NICU hospitalization, the individual studies were characerized by multiple methodological inadequacies (Table 1), limiting their ability to inform evidence-based NICU practice. In the absence of rigorous and compelling research about the effectiveness of MOM in reducing these serious and costly morbidities, hospital administrators were reluctant to invest economically in the NICU infrastructure required for the acquisition, storage, and feeding of MOM.
LOVE MOM Cohort
The LOVE MOM (Longitudinal Outcomes of VLBW Infants Exposed to Mothers’ Own Milk) cohort enrolled 430 very low birth weight (VLBW) infants (95% of eligible subjects) between 2008 and 2012 to study the relationship between the dose and exposure period of MOM feedings during NICU hospitalization and the risk of NEC, sepsis, and BPD, and their associated NICU costs. (National Institutes of Health, NR010009, Meier, PI). This prospective cohort study was designed to address the limitations in Table 1, with the exception of the inability to randomize to feeding groups.Central to the conceptualization of this study was determining which dose of MOM over which critical exposure periods during the NICU hospitalization reduces the risk of the specific morbidity. These morbidities occur at different ages after birth, are multifactorial, and may be differentially affected by specific MOM mechanisms or components [12]. For example, VLBW infants are at the greatest risk for NEC during the very early period after birth, and a key mechanism in the pathogenesis of NEC is local inflammation in the gut epithelial border [13]. Thus, we speculated MOM may reduce NEC via the array of growth factors, anti-inflammatory components, and other protective components in the early MOM produced by mothers who deliver prematurely [8]. Similarly, when modeling the effect of MOM on these morbidities, there is potential for reverse causality in which the dose of MOM is reduced due to the presence of the morbidity. For example, an early-onset morbidity such as NEC is managed clinically by placing the infant NPO (nil per os; not being fed enterally), so MOM is not fed during this time. If, as the independent variable, MOM is measured after the morbidity occurs, the low MOM intake may be a result of the morbid- ity versus a factor that contributed to its occurrence.
Although several studies had examined the contribution of MOM to neurodevelopmental outcome in premature, low birth weight (LBW), and extremely LBW (ELBW) infants (but not in VLBW infants) at the time the LOVE MOM cohort was created [11], follow-up through to 20 months of age, corrected for prematurity (CA) was not a part of the original NIH-funded study. Instead, the research on neurodevelopmental outcome in LOVE MOM was funded separately and included a subset of 251 smaller and sicker LOVE MOM cohort infants [14].
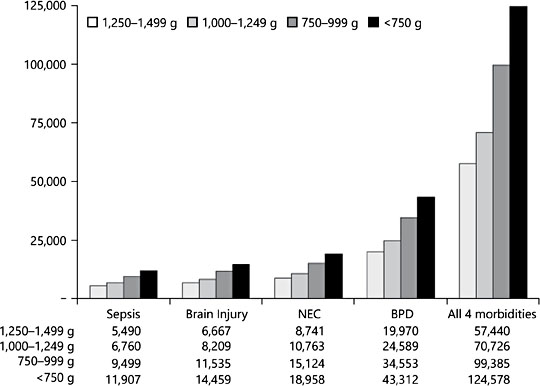
Fig. 1. Marginal costs of morbidities by birth weight, adjusted for infant sociodemographic characteristics [10, used with permission].
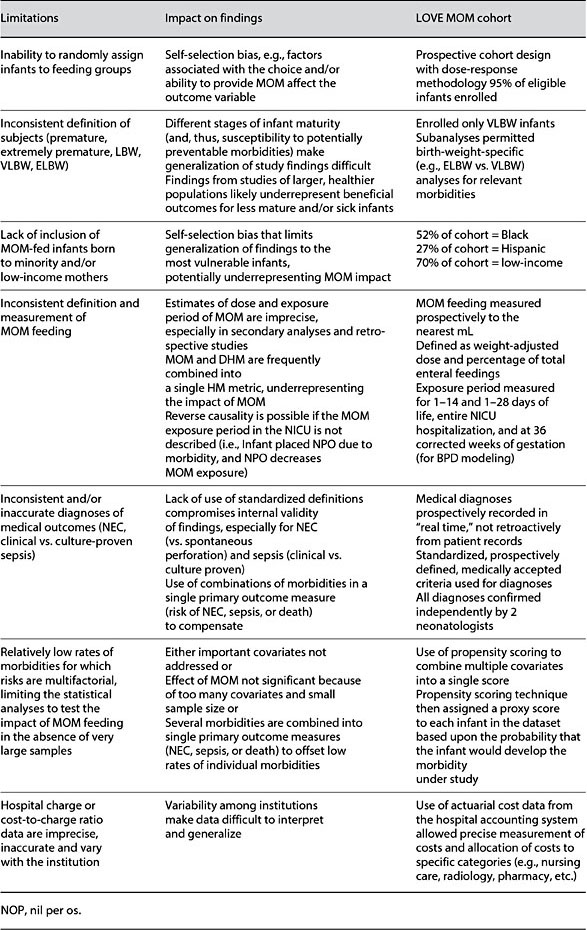
Table 1. Limitations in research addressing the impact of MOM feedings on health out- comes for premature infants
Subjects
LOVE MOM inclusion criteria were: birth weight <1,500 g; gestational age ≤35 weeks; if multiple birth, one infant selected randomly to participate; absence of congenital anomalies that might influence enteral feedings and/or cost of care; inborn or transferred to the Rush NICU within 24 h after birth; fed enterally within 14 days after birth; and negative maternal drug screen. Although maternal initiation of lactation was not an inclusion criterion, 98% of infants received some MOM. Table 2 summarizes the characteristics of the LOVE MOM cohort and their mothers. Unlike previous studies in this area, the sample consisted primarily of infants born to minority, low-income mothers who are the most likely in the United States to give birth to VLBW infants, but the least likely to initiate and maintain lactation [15].
Measures
The independent and dependent variables for this study have been detailed in original research papers and are summarized briefly here. All measures were collected prospectively and entered into the study database.
Dose and Exposure Period of MOM. Parenteral and enteral feedings were advanced according to a standardized protocol, and bovine-based powdered fortifier was added to MOM feedings when enteral feeding volume reached 140 mL/ kg/day. No DHM was used during this study, so infants received either fortified MOM, formula, or a mixture of both. Colostrum was fed in the order produced by the mother through to the achievement of full enteral feedings; thereafter, fresh MOM was prioritized over frozen MOM [16–18].
During each day of the NICU hospitalization, the milliliters of MOM and commercial formula were summed. These figures were used to calculate both a weight-adjusted MOM daily dose (HM-DD; mL/kg/day) and the percent of en- teral feedings equal to MOM (HM-PCT; mL MOM/[mL MOM + mL formula]). These MOM doses were calculated for 3 specific NICU exposure periods after birth: first 14 days of life (DOL); first 28 DOL (including first 14 days); and the entire NICU hospitalization [12]. For modeling of BPD, MOM dose was also calculated when the infant reached 36 weeks postmenstrual age because BPD is diagnosed at this time point.
Acquired Medical Morbidities. Specific criteria used for the diagnosis of NEC,sepsis, BPD, and neurodevelopmental problems were consistent with standard definitions in the literature and are detailed in the original publications. Each medical diagnosis was confirmed independently by 2 neonatologists.
NICU Cost of Care. Institutional costs were calculated by summing the direct cost of care for each chargeable item using the institution’s system-wide cost ac- counting system and combining this figure with indirect costs to yield total institutional costs. Provider costs were analyzed separately, and total costs were adjusted to the year in which the original papers were published [16–18].
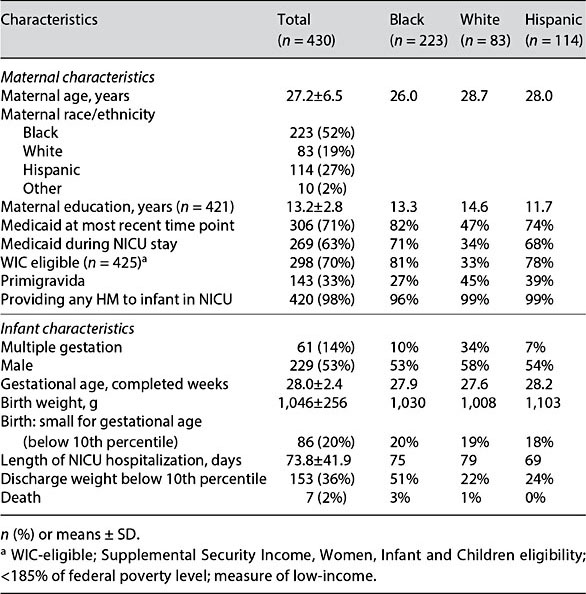
Table 2. Characteristics of the LOVE MOM cohort
Statistical Approaches
The overall incidence of these acquired morbidities is low, limiting the statistical analyses that can be used to model the risk of the morbidity as a function of MOM feedings. In the United States, NEC occurs in 5–7% of VLBW infants; sepsis in 22%, and BPD in 26–33% [19]. Additionally, these morbidities are considered multifactorial with numerous covariates including infant birth weight, gestational age, gender, duration of total parenteral nutrition, the day that enteral feedings were initiated after birth, use of prophylactic antibiotics beyond 48 h after birth, blood transfusion, ventilatory requirements, and others factors specific to the morbidity. Thus, controlling for the effect of these covariates on the outcome morbidity with a reasonable sample size has been a problem throughout MOM research with this population [20]. Our study incorporated propensity scoring to combine the covariates for each morbidity into a single risk score, which was then applied to each infant in the dataset as a proxy variable for the combination of covariates [21]. This technique permitted modeling the risk of the morbidity with a reasonable sample size, while controlling statistically for covariates.
Results
A dose-response relationship was demonstrated between the dose of MOM and a reduction in the risk of NEC, sepsis, BPD, and neurodevelopmental problems, with higher doses of MOM translating into lower risks and associated costs for the specific morbidity [14, 16–18]. Each morbidity and its associated cost reduction was linked to high-dose MOM during a specific critical exposure period during the NICU hospitalization, as detailed in Table 3.
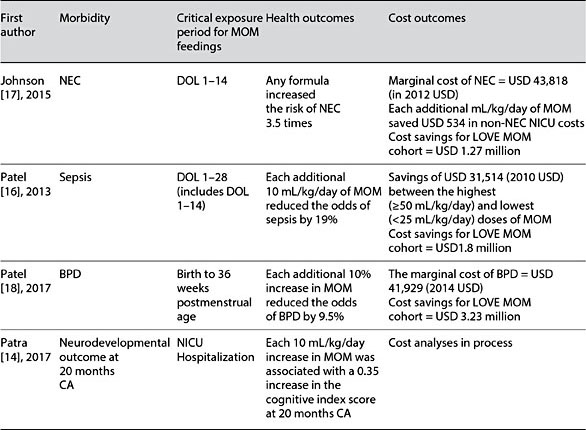
Table 3. Relationship between MOM dose and exposure and potentially preventable morbidities and their costs: the LOVE MOM cohort
Discussion
To the best of our knowledge, this is the first prospective study to report both the health and cost outcomes of MOM feedings in a contemporary, heterogeneous cohort of VLBW infants during the NICU hospitalization and again at 20 months CA. Cumulatively, these 4 publications reveal a dose-response relationship between the volumes of MOM received during critical exposure periods in the NICU and a reduction in the risk of the specific morbidity and its associated costs. Furthermore, our 20-month data indicate that high-dose MOM feedings through to NICU discharge significantly decreased the risk of neurodevelopmental problems in a subset of the smallest, sickest infants in the LOVE MOM cohort.
Necrotizing Enterocolitis
Three separate studies, a prospective [22], a retrospective [23], and a secondary analysis of an existing dataset [24], have reported similar findings about the reduction in NEC with high-dose MOM feedings during the early period after birth. In a prospective study, Sisk et al. [22] reported that odds of NEC were decreased 6-fold in VLBW infants who received ≥50% of their enteral feed volume as MOM during the first 14 DOL. In a retrospective analysis of 349 VLBW infants, Corpeleijn et al. [23] reported similar findings for the per- centage of MOM received during the first 10 DOL and a reduction in the combined variable of NEC, sepsis, or death. In a secondary analysis of data from 1,272 ELBW infants enrolled in the NICHD-funded multisite glutamine trial, Meinzen-Derr et al. [24] reported a dose-response relationship between the percentage of enteral feedings equal to MOM during the first 14 DOL and a reduction in the risk of either NEC or death after 14 DOL, with a trend towards the greatest reduction being afforded by 100% MOM feedings.
Whereas multiple bioactive components in early MOM colostrum and transitional milk provide support for growth, protection and maturation of the vulnerable gut epithelial border during the transition from intra- to extrauterine nutrition [25, 26], another likely mechanism of protection, is the avoidance of bovine protein during this critical window after birth [25, 26]. Bovine protein upregulates local inflammatory processes and compromises the integrity of the gut epithelial border potentiating the translocation of high-molecular weight pathogens and their toxins into the underlying mucosa [27–29]. The fact that DHM also reduces the risk of NEC but has no favorable impact on other potentially preventable complications underscores the plausibility that bovine-based feedings contribute separately to NEC [26].
Sepsis
Similar to our findings, Furman et al. [30] reported a dose-response relation- ship between the dose of MOM feedings (≥50 and 25–49.99 mL/kg/day) received during the first 28 DOL and reduction in sepsis for VLBW infants. Whereas multiple other studies have reported a reduction in the risk of sepsis with MOM feedings, major limitations are whether sepsis was measured as clinical or culture-proven and whether the potential for reverse causality was addressed [31].
The findings that MOM feedings do not need to be exclusive in order to reduce the risk of sepsis, and that a lesser dose of 25–49.99 mL/kg/day of MOM affords some protection suggest that the bioactive components in MOM rather than the avoidance of bovine-based products is a major mechanism of protection. This speculation is supported by the findings that DHM affords no reduction in the risk of sepsis in this population [26]. Likely bioactive components include anti-infectives such as secretory IgA, a MOM proteome that targets immunomodulation, MOM-borne probiotic bacteria, and oligosaccharides with prebiotic activity [16, 25]. Additionally, sepsis is linked with multiple fac- tors that are indirectly affected by MOM feedings such as shorter duration of central-line catheters and parenteral nutrition, both of which increase the risk of sepsis [16].
Bronchopulmonary Dysplasia
Only one previous multicenter cohort study has compared the rates of BPD for exclusively HM-fed (mostly MOM with some DHM; n = 223) and exclusively formula-fed (n = 249) VLBW infants, reporting significantly lower rates of BPD in exclusively HM-fed infants [32]. These findings are remarkably similar to ours, with the exception that Spiegler et al. [32] compared only the two extremes of MOM dose (exclusive or none), whereas we reported a dose-response relationship with each additional 10% of enteral feedings between birth and 36 weeks postmenstrual age reducing the odds of BPD by 9.5%. Of particular inter- est is that both studies found slightly slower rates of in-NICU weight gain with high-dose MOM feedings, suggesting that there may be a trade-off between reducing BPD and achieving weight gain targets in VLBW infants [18, 32].
Although BPD is much less studied than other potentially preventable morbidities, MOM feedings provide recipient infants with antioxidant [33] and anti-inflammatory [34] components as well as myoinositol [35], which has been linked with a reduction in BPD when used as a supplement in this population [36]. Additionally, MOM may provide protection from BPD indirectly by reducing the risk of NEC and sepsis, both of which upregulate inflammation and have been associated with the subsequent development of BPD [18].
Neurodevelopmental Outcome at 20 Months Corrected Age
Previous studies have examined the relationship between MOM feedings and neurodevelopmental outcome in premature infants, but all have been characterized by the multiple limitations outlined in Table 1 [11]. To our knowledge, only our study included meticulous prospectively collected information about the NICU dose and exposure period of MOM feedings in a contemporary co- hort of VLBW infants. Our findings revealed a significant dose-response relationship, with each additional 10 mL/kg/day MOM intake during the NICU hospitalization associated with an additional 0.35 cognitive index score. This difference is equal to a 5-point difference between our lowest and highest MOM dosing groups, despite the fact that infants in the highest quintile of MOM dose gained weight slightly less rapidly and were more likely to be discharged below the 10th percentile for weight (extrauterine growth retardation; EUGR) than infants in lower MOM dosing quintiles. In bivariate analyses, EUGR was a significant risk factor for neurodevelopmental problems, but in multivariate anal- yses EUGR was mitigated by high-dose MOM feedings. In other words, if the slightly slower weight gain during the NICU hospitalization is a function of high-dose MOM feedings, our findings suggest that high-dose MOM feedings should be prioritized.
In addition to providing nutritional substrate to optimally support the high metabolic activity and growth of the human brain, bioactive MOM components provide neuroprotection to the vulnerable, rapidly growing white matter in the premature infant brain, the integrity of which has been linked to subsequent neurodevelopmental outcome in several studies [5, 37–40]. Furthermore, NEC, sepsis, and BPD increase the risks of neurodevelopmental problems in premature infants, so the fact that MOM feedings reduce these NICU morbidities contributes indirectly to neurodevelopmental outcome differences [14]. In a large randomized trial of MOM feedings supplemented with either DHM or formula in VLBW infants, DHM feedings had no beneficial impact on neurodevelopmental outcome [41].
Conclusion
High-dose MOM feedings during 3 critical exposure periods in the NICU hospitalization provide significant reductions in the risk of NEC, sepsis, BPD, and neurodevelopmental problems in VLBW infants. These same outcomes are not achievable with either DHM or infant formula. Furthermore, the reduction in these morbidities translates into significant cost savings, and findings support the economic investment into the NICU infrastructure that is necessary for the acquisition, storage, and feeding of MOM.
References
- 1 Budin P: The Nursling: The Feeding and Hygiene of Premature and Full-Term Infants. London, Caxton, 1907.
- 2 Hess JH, Lundeen EC: The Premaure Infant: Its Medical and Nursing Care. Philadelphia, Lippincott, 1941.
- 3 Hepner WR Jr, Krause AC: Retrolental fibroplasia: clinical observations. Pediatrics 1952; 10:433–443.
- 4 Lucas A, Gore SM, Cole TJ, et al: Multicentre trial on feeding low birthweight infants: effects of diet on early growth. Arch Dis Child 1984;59:722–730.
- 5 Isaacs EB, Fischl BR, Quinn BT, et al: Impact of breast milk on intelligence quotient, brain size, and white matter development. Pediatr Res 2010;67:357–362.
- 6 Lucas A: Programming by early nutrition: an experimental approach. J Nutr 1998;128(2 suppl):401S–406S.
- 7 Miracle DJ, Meier PP, Bennett PA: Mothers’ decisions to change from formula to mothers’ milk for very-low-birth-weight infants. J Ob- stet Gynecol Neonatal Nurs 2004;33:692–703.
- 8 Meier PP, Engstrom JL, Patel AL, et al: Improving the use of human milk during and after the NICU stay. Clin Perinatol 2010;37:217–245.
- 9 Meier PP, Johnson TJ, Patel AL, Rossman B: Evidence-based methods that promote human milk feeding of preterm infants: an expert review. Clin Perinatol 2017;44:1–22.
- 10 Johnson TJ, Patel AL, Jegier BJ, et al: Cost of morbidities in very low birth weight infants. J Pediatr 2013;162:243–249.e1.
- 11 Lechner BE, Vohr BR: Neurodevelopmental outcomes of preterm infants fed human milk: a systematic review. Clin Perinatol 2017;44: 69–83.
- 12 Bigger HR, Fogg LJ, Patel A, et al: Quality indicators for human milk use in very low- birthweight infants: are we measuring what we should be measuring? J Perinatol 2014;34: 287–291.
- 13 Patel AL, Panagos PG, Silvestri JM: Reducing incidence of necrotizing enterocolitis. Clin Perinatol 2017;44:683–700.
- 14 Patra K, Hamilton M, Johnson T, et al: NICU human milk dose and 20-month neurodevel- opmental outcome in very low birth weight infants. Neonatology 2017;112:330–336.
- 15 Engstrom JL, Patel AL, Meier PP: Eliminating disparities in mother’s milk feeding in the neo-natal intensive care unit. J Pediatr 2017;182:8–9.
- 16 Patel AL, Johnson TJ, Engstrom JL, et al: Im- pact of early human milk on sepsis and health care costs in very low birthweight infants. J Perinatol 2013;33:514–519.
- 17 Johnson TJ, Patel AL, Bigger HR, et al: Cost savings of human milk as a strategy to reduce the incidence of necrotizing enterocolitis in very low birth weight infants. Neonatology 2015;107:271–276.
- 18 Patel AL, Johnson TJ, Robin B, et al: Influence of own mother’s milk on bronchopulmonary dysplasia and costs. Arch Dis Child Fetal Neonatal Ed 2017;102:F256–F261.
- 19 Horbar JD, Edwards EM, Greenberg LT, et al: Variation in performance of neonatal intensive care units in the United States. JAMA Pediatr 2017;171:e164396.
- 20 Ip S, Chung M, Raman G, et al: Breastfeeding and Maternal and Infant Health Outcomes in Developed Countries. Evidence Report/Technology assessment No. 153 (prepared by Tufts-New England Medical Center Evidence-based Practice Center, under contract No. 290-02-0022). AHRQ Publication No.07-E007. 2007.
- 21 Rosenbaum PR, Rubin DB: The central role of the propensity score in observational studies for causal effects. Biometrika 1983;70:41–55.
- 22 Sisk PM, Lovelady CA, Dillard RG, et al: Early human milk feeding is associated with a lower risk of necrotizing enterocolitis in very low birth weight infants. J Perinatol 2007;27: 428–433.
- 23 Corpeleijn WE, Kouwenhoven SM, Pappa MC, et al: Intake of own mother’s milk during the first days of life is associated with decreased morbidity and mortality in very low birth weight infants during the first 60 days of life. Neonatology 2012;102:276–281.
- 24 Meinzen-Derr J, Poindexter B, Wrage L, et al: Role of human milk in extremely low birth weight infants’ risk of necrotizing enterocolitis or death. J Perinatol 2009;29:57–62.
- 25 Meier PP, Patel AL, Bigger HR, et al: Human milk feedings in the neonatal intensive care unit; in Rajendram R, Preedy VR, Patel VB (eds): Diet and Nutrition in Critical Care. New York, Springer, 2015, pp 807–822.
- 26 Meier P, Patel A, Esquerra-Zwiers A: Donor human milk update: evidence, mechanisms, and priorities for research and practice. J Pediatr 2017;180:15–21.
- 27 Caicedo RA, Schanler RJ, Li N, Neu J: The developing intestinal ecosystem: implications for the neonate. Pediatr Res 2005;58:625–628.
- 28 Taylor SN, Basile LA, Ebeling M, Wagner CL: Intestinal permeability in preterm infants by feeding type: mother’s milk versus formula. BreastfeedMed 2009;4:11–15.
- 29 Penn A: Digested formula but not digested fresh human milk causes death of intestinal cells in vitro: implications for necrotizing enterocolitis. Pediatr Res 2012;72:560–567.
- 30 Furman L, Taylor G, Minich N, Hack M: The effect of maternal milk on neonatal morbidity of very low-birth-weight infants. Arch Pediatr Adolesc Med 2003;157:66–71.
- 31 de Silva A, Jones PW, Spencer SA: Does human milk reduce infection rates in preterm infants? A systematic review. Arch Dis Child Fetal Neonatal Ed 2004;89:F509–F513.
- 32 Spiegler J, Preuss M, Gebauer C, et al: Does breastmilk influence the development of bronchopulmonary dysplasia? J Pediatr 2016; 169:76–80.e4.
- 33 Friel J, Diehl-Jones B, Cockell K, et al: Evidence of oxidative stress in relation to feeding type during early life in premature infants. PediatrRes 2011;69:160–164.
- 34 Collado MC, Santaella M, Mira-Pascual L, et al: Longitudinal study of cytokine expression, lipid profile and neuronal growth factors in human breast milk from term and preterm deliveries. Nutrients 2015;7:8577–8591.
- 35 Pereira GR, Baker L, Egler J, et al: Serum myoinositol concentrations in premature in- fants fed human milk, formula for infants, and parenteral nutrition. Am J Clin Nutr 1990;51:589–593.
- 36 Howlett A, Ohlsson A, Plakkal N: Inositol in preterm infants at risk for or having respiratory distress syndrome. Cochrane Database Syst Rev 2015;2:CD000366.
- 37 Kapellou O, Counsell SJ, Kennea N, et al: Ab- normal cortical development after premature birth shown by altered allometric scaling of brain growth. PLoS Med 2006;3:e265.
- 38 Keunen K, van Elburg RM, van Bel F, Bend- ers MJ: Impact of nutrition on brain development and its neuroprotective implications following preterm birth. Pediatr Res 2015;77: 148–155.
- 39 Woodward LJ, Clark CA, Bora S, Inder TE: Neonatal white matter abnormalities an important predictor of neurocognitive outcome for very preterm children. PLoS One 2012; 7:e51879.
- 40 Sherman MP, Zaghouani H, Niklas V: Gut microbiota, the immune system, and diet influence the neonatal gut-brain axis. Pediatr Res 2015;77:127–135.
- 41 O’Connor DL, Gibbins S, Kiss A, et al: Effect of supplemental donor human milk com- pared with preterm formula on neurodevelopment of very low-birth-weight infants at 18 months: a randomized clinical trial. JAMA 2016;316:1897–1905.