Guiding Development of the Neonate: Lessons from Mammalia
Abstract
Significantly preterm and low-birthweight (LBW) babies have diminished lung and gut development, generally fail to thrive, have increased mortality and higher frequency of mature-onset disease. Mothers often cannot breastfeed, and babies receive either formula or pasteurized donor milk, which may further limit the baby’s recovery. New approaches are required to manage the early stages of neonatal development. The tammar wallaby, an Australian marsupial, has a short gestation and a simple placenta, and gives birth to an altricial young equivalent to a final trimester human embryo. The neonate remains in the pouch and attached to the teat for 100 days postpartum. The mother slows growth of the young and progressively changes the composition of the milk to deliver signals for organ development, including the lung and gut. This closely resembles the relationship between the human fetus and delivery of placental and uterine bioactives. Datasets comprised of differentially expressed genes coding for secreted proteins in early lactation in the tammar mammary gland have been compared to data- bases produced from human placenta, amniotic fluid, colostrum and milk to identify human homologues for the putative signaling molecules for organ development. These data will be used to develop milk fortifiers for treatment of preterm and LBW babies in both the developed and the developing world.
Introduction
Significantly preterm and low-birthweight (LBW) babies have acute challenges for survival, largely due to limited development of their lungs and gut. Furthermore, disruption to the timing of developmental programming in the neonate often results in increased frequency of mature-onset disease, and this is exacerbated if growth rates are accelerated too aggressively [1]. The cost to manage these babies in hospitals is considerable, and there is an increased prevalence of this problem in the developing world. Mothers often cannot breastfeed, and the only option available is providing either formula or pasteurized donor milk to improve the development of these babies. New approaches are required to manage the early stages of treatment, and particularly a focus on the development of organs without an accompanying large increment in growth.
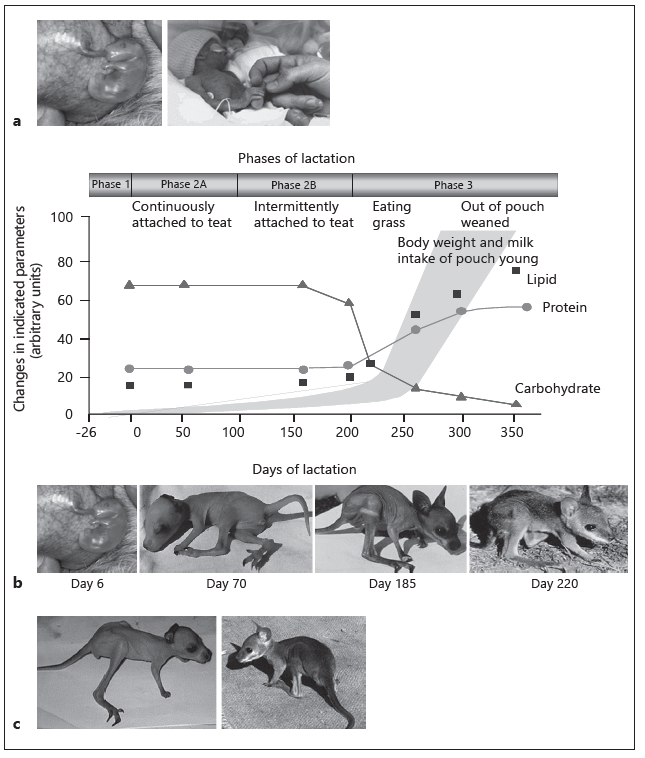
Milk has multiple functions. Evolutionary pressure on mammals has ensured that milk provides the most appropriate nutrition for growth of the newborn. It also has the capacity to remodel the mammary gland and can provide protection from infection and inflammation when the mammary gland is susceptible to these challenges. Innovative studies are now exploiting the unusual reproductive strategy of an Australian marsupial, the tammar wallaby (Macropus eugenii) to identify milk bioactives that impact on tissue development in the neonate [2, 3]. Reproduction in the tammar wallaby is characterized by a short 26-day gestation, a simple placenta [4], birth of an immature young, and a relatively long 300-day lactation (Fig. 1a, b). During the first 100 days postpartum, the development of the neonate is similar to that of a eutherian fetus in its final trimester [2, 3].
Both the tammar newborn and the human preterm baby will receive milk to sustain their development. The preterm baby will continue to face serious acute and chronic health challenges that may limit a timely recovery. In contrast, the tammar neonate will receive milk that is appropriate for growth and development of a healthy young. Therefore, can an understanding of the milk bioactivity provided to the tammar neonate underpin new approaches to develop a human milk fortifier to better manage preterm babies?
During early lactation in the tammar, the immature organs that are necessary for their survival such as respiratory system, gut, lymphoid tissues, and nervous system, including brain and spinal cord, are rapidly developed [5–9]. The major and minor milk constituents change substantially and progressively during lactation in the tammar, and these changes have been shown to regulate growth and development of the tammar pouch young, allowing specific changes in milk composition to be correlated with specific developmental events [2, 3]. Interestingly, during the first 100 days of lactation, the mother delivers milk with a composition that slows growth of the young but augments an increased rate of organ development [10]. Transferring young to a mother at a more advanced stage of lactation accelerates this process and supports the argument that exposure of the young to a more mature milk with a range of new bioactivity has the potential to impact on development of the fostered young (Fig. 1d) [6, 11, 12]. Therefore, the signaling factors involved in the development of the eutherian fetus, most of which would be derived from the placenta, amniotic fluid, and perhaps colostrum, are most likely delivered in the milk of marsupials [6, 11, 12].
Since the appearance of the aplacental, egg-laying monotremes 200 million years ago, there has been extensive adaptation to reproduction, particularly in lactational strategies when the Theria split into the Metatheria (Marsupialia) and Eutheria (Placentalia) lineages over 140 million years ago [13]. In contrast to marsupials, eutherians have a well-developed placenta and a long gestation that leads to the birth of a relatively well-developed young. The length of lactation is often similar to gestation, and composition of the milk does not change substantially. This is consistent with the concept that signaling molecules are presented by the placenta and amnion prior to parturition. Therefore, a strong argument is growing for a comparative approach to better identify the specific signals that regulate development. The regulatory mechanisms controlling the great majority of physiological processes have been conserved during evolution, but the timing and mechanism for delivering these processes may differ between species of mammals [2, 3]. Therefore, the use of these diverse species, coupled with the availability of current technologies, provides the opportunity to exploit marsupial models for new insights into the functions of milk. This may lead to a new range of human fortifiers that include bioactives with the potential to specifically target the growth and development of tissues in the human neonate to improve outcomes for premature and LBW babies.
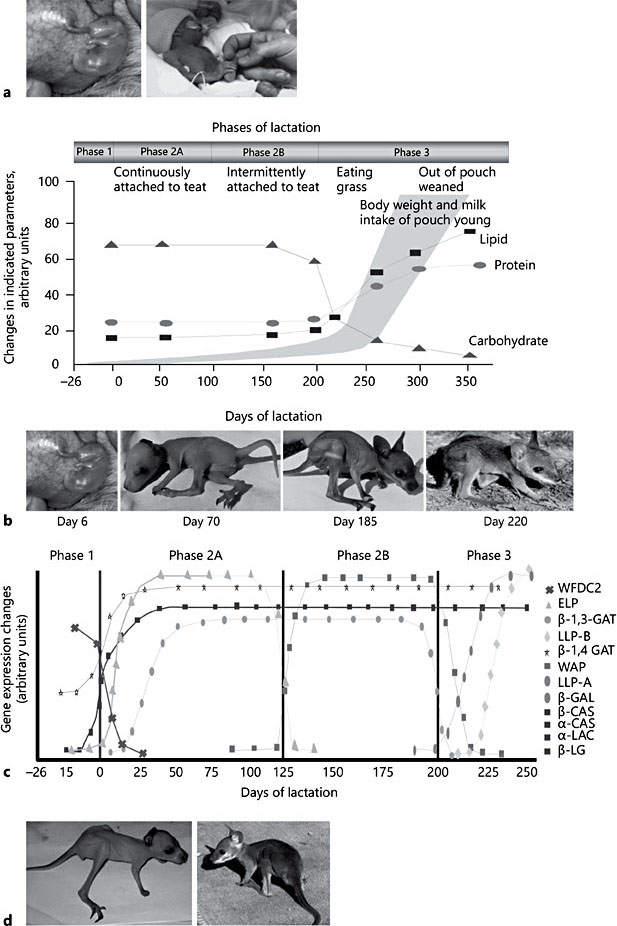
Fig. 1. a The tammar as a model system for premature and low-birthweight babies. The tammar young is 6 days of age, the human neonate is 26 weeks of age. b Tammar wallaby lactation strategy. Progressive changes in milk composition and growth of the young during the three phases of the lactation cycle in the tammar wallaby. Total protein concentration does not change significantly during early lactation but there is a progressive change in the kinds of proteins secreted. c Differential expression of the major milk proteins during tammar lactation. The profile of α-casein (α-CAS), β-casein (β-CAS), α-lactalbumin (α-LAC), β-lactoglobulin (β-LG), early lactation protein (ELP), whey acidic protein (WAP), late lactation protein-A (LLP-A), and late lactation protein-B (LLP-B) gene expression. d Fostered pouch young. Pouch young at day 120 of age were either cross- fostered to host mothers at day 170 of lactation or retained on mothers at day 120 of lactation. After 50 days, both pouch young were removed. The more mature animal (shown on the right) was fostered to a mother at a more advanced stage of lactation. Adapted from Sharp et al. [3].
Regulation of the Tammar Lactation Cycle
The tammar lactation cycle is divided into four broad phases (Fig. 1b) [2, 3, 10]. Phase 1 is comprised of a 26-day gestation including the subsequent birth of the altricial young which climbs into the pouch and attaches to one of four teats. During the first 100 days postpartum (phase 2A), the immature neonate remains permanently attached to the teat and has limited capacity to mount an immune response [7]. The mother produces relatively small volumes of dilute milk containing a high concentration of complex carbohydrates and a low concentration of protein and lipid (Fig. 1b). The conversion of milk to body mass during this phase is similar to that reported for a eutherian fetus [10]. Phase 2B commences 100 days postpartum and continues for approxi- mately 100 days during which the neonate remains in the pouch, relinquish- es the teat, and continues to suckle intermittently. The milk produced is maintained with high levels of carbohydrates and low concentrations of protein and lipids until the onset of phase 3 when the young begins to exit the pouch and feeds on herbage, but continues to suckle. During phase 3, the mammary gland enlarges significantly, producing large amounts of milk that is rich in protein and lipid but low in carbohydrates to provide a high-energy milk. Recent experiments have shown that the lactation program, and particularly these changes in milk composition are regulated by the mammary extracellular matrix [14].
During tammar lactation, casein, α-lactalbumin, and β-lactoglobulin genes are induced at parturition and remain expressed throughout lactation which is similar to the eutherian mammals. However, there are significant temporal changes in expression of some of the major milk protein genes and many of the minor milk protein genes (Fig. 1c) [2]. In addition, microarray analysis of the tammar mammary gland has revealed a multitude of changes in gene expression during the lactation cycle [2]. The tammar-specific microarray was generated from a normalized mammary gland cDNA library produced using an expression vector. Therefore, specific proteins could be synthesized in vitro following transfection of the plasmid into CHO cells [2] and secretion of the protein into the culture media. This process produced enough protein to examine bioactivity in any relevant in vitro model and subsequently revealed temporal delivery of a range of bioactivities. The microarray analysis also confirmed expression of some major milk proteins expressed in each phase of lactation that were used as markers to examine the regulation of the lactation program in the mammary gland [2, 14].
The Role of Milk Bioactives in Development of Specific Tissues in the Suckled Tammar Neonate
Development of the Gut
Previous studies have shown dramatic changes in gut morphology occur in the suckled young and take place while the young is still in the pouch [6, 15]. In the hindstomach region, parietal cells increase in number, gastric glands enlarge and adopt the adult-like phenotype, and peptic enzyme activity becomes elevated. Concomitantly, the forestomach region changes from an immature gastric glandular phenotype to a cardia glandular phenotype in the region that becomes the adult forestomach. These changes in stomach morphology were correlated with significant changes in milk composition, and a study that transferred pouch young to host mothers at a more advanced stage of lactation for 50 days indicated that the process of forestomach maturation was accelerated [6].
More recent studies have shown that milk collected from tammars in the first 100 days of lactation and cultured with stomach explants from day 12 mouse embryos resulted in elevated cell proliferation and increased level of expression of specific developmental gene markers [Kurappath et al., unpubl.]. Therefore, identification of these developmental signaling molecules in tammar milk will show promise for new strategies to address limited gut development.
Development of the Lung
In eutherians, the majority of lung morphogenesis occurs during gestation to enable gaseous exchange at birth. In contrast, studies of lung development in several marsupial species, including the tammar wallaby [3] have demonstrated that the major developmental changes in the respiratory system occur during early postnatal life [5]. A recent study using Monodelphis domestica (the American opossum) has shown that major postnatal development of the lung was similar to the tammar neonate. RNAseq analysis of the Monodelphis lung during each major stage of development was compared with RNAseq analysis of the mouse lung in embryos and suggested that the potential regulatory process- es were very similar despite the timing of this development in the neonate and fetus, respectively [Modepalli et al., unpubl.]. Therefore, it is likely the lung is signaled by a conserved mechanism provided by either the milk in marsupials or the uterine environment in eutherians.
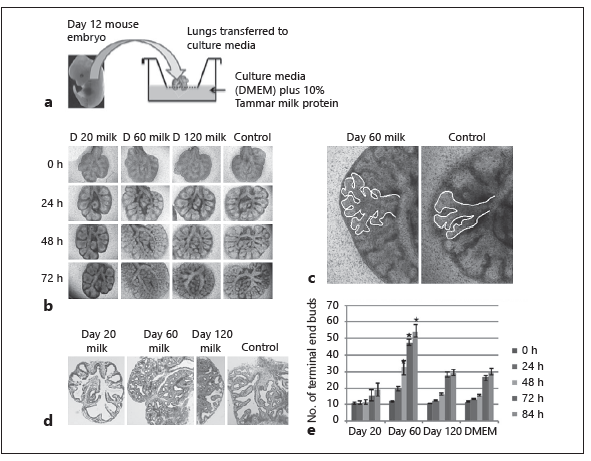
The potential role of tammar milk in lung development was examined using mouse embryonic lungs (E-12) cultured in media with tammar skim milk collected at key time points during lactation (Fig. 2a) [16]. The embryonic lungs showed increased branching morphogenesis when incubated with milk collected between day 40 and 100 of lactation, and reduced lung development when incubated in media with milk from day 20 lactation (Fig. 2b–d) [16]. In addition, day 60 milk significantly upregulated a number of marker genes for key developmental processes and specialized cell types including the potential to increase production of surfactant (Fig. 2f) [16]. This temporal effect was lost in milk collected from day 100 to 200 of lactation.
The mechanisms by which the day 60 milk stimulated lung development were examined further [16] to show that lung epithelial cells cultured on Matrigel in media with day 60 milk proteins had increased proliferation and formed organoid structures with a lumen. In separate experiments, the lung mesenchymal cells cultured on Matrigel showed increased proliferation and invaded the surrounding matrix with epithelial branching morphogenesis [16]. The mesenchymal cells became flattened, elongated, and spindle shaped, with a similar morphology to either airway smooth muscle cells or myofibroblast cells.
Cross-fostering experiments have been used to assess effects of milk composition on lung development in the tammar [17]. The pouch young at day 25 of age were fostered to a series of mothers at day 15 of lactation, so that the young only received milk from day 15 to 25 of lactation for a period of 20 days. Analysis showed that expression of marker genes related to branching morphogenesis, alveolization, and presence of terminal and airway epithelia were significantly reduced in lungs from fostered pouch young compared to lungs from control pouch young. These data are consistent with a diminished level of differentiation of the lung in fostered young.
Taken collectively, the analysis of lung development identified a window of bioactivity in milk samples collected between day 20 and day 100 of lactation. Subsequent analysis of proteins in milk at day 20, day 60, and day 120 of lactation using mass spectrometry identified 19 proteins, predominately growth factors that have the potential to directly stimulate lung development [17].
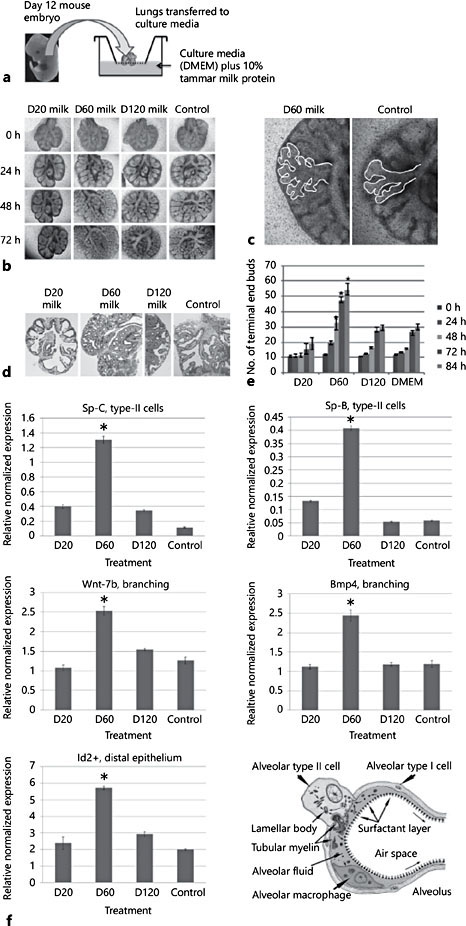
Fig. 2. Effect of early-phase tammar wallaby milk on lung branching morphogenesis. a Lungs were removed from embryonic mice and cultured with 10% tammar milk. b–e Lungs showed extensive branching morphogenesis and increased volume after 3 days of culture with milk collected at day (D) 60 of lactation but not lungs cultured in media with either 10% PBS (control) or day 120 milk. Sections of lung stained with HE confirmed that the morphology of embryonic lungs treated with day 60 milk showed increased branching. f Effect of early-phase tammar wallaby milk on lung developmental gene markers. Marker genes for lung development were all significantly upregulated in lung cultured with day 60 milk. Expression of Sp-B and Sp-C genes indicated increased type II cells producing surfactant, wnt-7b and BMP4 gene expression increased confirming branching morphogenesis, and increased Id-2 gene expression confirmed cell proliferation and growth of the lung. Adapted from Sharp et al. [3]. * p < 0.005.
Mechanisms for Delivery of Bioactivity in Milk
The major bioactives in milk fall within a number of groups: proteins, peptides, complex carbohydrates, and miRNA. The proteins and peptides are of particular interest given that bioinformatics analysis of the tammar mammary transcriptome has revealed more than 100 secreted proteases and an almost equal number of secreted protease inhibitors [Watt et al., unpubl.]. This provides a very complex set of interactions and the potential for a huge repertoire of proteins and peptides that can be delivered temporally in the milk. In addition, there are ex- amples of alternative splicing of mammary genes to deliver domain-specific proteins in a timed way to impact potentially on the mammary gland and the development of the young as required [2].
The tammar has also provided an interesting model to better assess the potential of milk miRNA delivered in exosomes to signal events in the young. The kinds of miRNA in tammar milk change during lactation, and there is evidence that some of the miRNA appear to be produced in the mammary gland, and can be transferred across the gut to the peripheral circulation in the suckled young. Therefore, it is likely milk miRNA represent not only potential markers of mam- mary gland development and activity during the lactation cycle, but also new putative signaling molecules involved in programming development of the suckled young [18].
Human Milk Bioactivity: Acute Response to Challenge and Potential for Breast-Programmed Development
The capacity of human colostrum and milk to signal development of tissue has not been extensively explored. However, breast milk does have the capacity to respond rapidly to short-term changes to breastfeeding without the need for any metabolic or genomic intervention. Recent experiments examined skim milk prepared from samples collected from women in mid-lactation and incubated at 37°C over a period of 7 days to allow digestion of milk protein by endogenous proteases [Watt et al., unpubl.]. Results showed peptides were predominantly derived from caseins with limited digestion of the whey proteins. Subsequent antibacterial assays using Staphylococcus aureus, a major cause of breast infection showed the milk peptides significantly increased antimicrobial activity. The peptides also showed increased levels of anti-inflammatory activity [Watt et al., unpubl.]. Importantly, the peptides did not show any capacity to program apoptotic activity in human mammary epithelial cells. Therefore, the milk has an immediate capacity to produce peptides that play a specific role in the breast to reduce infection and inflammation if there is an interruption to breastfeeding.
A more extensive response is observed during mastitic challenge to the breast and can be assessed by analyzing cells in milk. A recent study [19] showed microarray analysis of genes expressed in cells present in breast milk at days 24, 48, and 101 of lactation, and days 7 and 14 of involution provided a reasonable snapshot of the secretory activity of the breast, despite some changes in the types of cells in milk. Analysis of cells in milk from women with mastitis identified increased expression of a new class of genes not expressed in lactation. Subsequently, treatment of human mammospheres in culture with bacterial LPS showed similar genes were expressed, confirming a response is evident after directly challenging the mammary epithelial cells [Watt et al., un- publ.]. However, this kind of acute response is necessary to protect the breast and to maintain lactation and is not aligned with the programmed delivery of bioactives that stimulates tissue development observed in marsupials. The prospect of using genome expression databases generated from the tammar mammary gland during development of the neonate may prove useful to interrogate datasets derived from human colostrum, milk, placenta, and amniotic fluid to better understand the signaling of development in the human fetus and neonate.
The most significant change in the composition of human milk that equates with changes seen in the transition between phases of lactation in marsupials is during the transition from colostrum to milk. Colostrum has been identified as a rich source of growth factors, immunoglobulins, and other proteins that are important to provide a “positive start” to development of the newborn baby. However, it is timely to reexamine the potential role of colostrum for its impact on the development of the baby, and particularly to explore any influence of colostrum on either initiating or augmenting the development of specific tissues. Therefore, it is particularly relevant to query databases from these sources in the comparison with genes exclusively and differentially expressed in the tammar mammary gland during early lactation.
A Comparison of Tammar and Human Databases to Identify Human Colostrum, Milk, Placental, and Amniotic Fluid Bioactives
To better understand the concept that expression of putative signaling molecules in phase 2A milk from the tammar may inform us about the role of the placenta, amniotic fluid, colostrum, and milk in the development of neonatal tissues in the human, a number of genomic databases have been compared. The mammary glands from a total of 20 wallabies at phase 2A, 2B, and 3 of lactation were analyzed using RNAseq and microarray [2], and genes expressed either exclusively in phase 2A or with expression levels greater than 2-fold (p < 0.05) higher than genes in phase 2B and 3 were identified. Cells from human milk collected either at day 30 of lactation or from colostrum were analyzed by either RNAseq [Geddes and Twigger, unpubl. data] or microarray [19]. The genes ex- pressed either exclusively or at levels 2-fold greater (p < 0.05) in cells from colostrum or milk were identified. Genomic datasets for human placenta and amniotic fluid were publically available and included in the study [1, 20]. Importantly, the only genes analyzed in this study were identified as coding for secreted proteins which potentially are made available to targets [2].
The first surprising observation was the identification of 105 genes common to the wallaby phase 2A mammary tissue and human colostrum. These genes are currently being analyzed further but indicate the potential of colostrum for having an impact on early development of the neonate, in addition to uterine-derived signal- ling. Additional datasets produced from the colostrum associated with significantly preterm and LBW babies will be essential to determine whether the same proteins are made available to term, preterm, and LBW babies. These data will be important not only to better understand the potential acute impact on the neonate but conceivably could be important for influencing the incidence of mature-onset disease that presumably is a consequence of the lack of signalling these events at a critical time point in the development of the baby. The comparisons of the tammar mammary gland and human placenta, and tammar mammary gland and the amniotic fluid identified additional genes present exclusively in the human dataset. These data show promise for identifying genes with the potential to regulate development of the human fetus and neonate and that may have been co-opted from the marsupial mammary gland.
Conclusion
The mammary gland in marsupial species is extremely sophisticated in terms of the molecular organization for temporal delivery of bioactives to multiple targets. Clearly, the role of the mammary extracellular matrix has evolved in eutherians and has retained the function to regulate morphology and differentiation of the mammary gland but no longer has a role in changing the composition of the milk. It is now emerging that the marsupial provides a unique opportunity to more easily identify the bioactives that potentially play a role in early development of the fetus and neonate.
These outcomes are important for development of better therapeutic options to treat the limited lung and gut development in premature and LBW babies that fail to thrive and that can have a significantly higher rate of mortality. This opportunity may extend to investigating the significant programming of develop- mental clocks that occurs in the earlier stages of development and that subsequently impacts on the increased prevalence of mature-onset disease seen in these babies [21]. It will be interesting to determine whether the developmental program is set in marsupials during the characteristically short gestation, which can be as little as 8 days [10], or whether the milk is providing signals postnatally to the altricial neonate. These models also provide the option of cross-fostering neonates to mothers at other stages of lactation to exclude windows of potential delivery of putative milk bioactives at critical times in the developmental process and to examine the impact of increased growth rates which must be managed to ensure a better outcome for acute and chronic treatment of premature and LBW babies.
References
- 1 Lee AC, Katz J, Blencowe H, et al: National and regional estimates of term and preterm babies born small for gestational age in 138 low-income and middle-income countries in 2010. Lancet Global Health 2013;1:e26–e36.
- 2 Sharp JA, Watt A, Bisana S, et al: The comparative genomics of marsupials, monotremes and pinnippeds; models to examine function of milk proteins; in Singh HA, Thompson HA, Boland M (eds): Milk Proteins: From Expression to Food. Amsterdam, Elsevier, 2014, pp 76–112.
- 3 Sharp JA, Wanyonyi S, Modepalli V, et al: The tammar wallaby: a marsupial model to examine the timed delivery and role of bioactives in milk. Gen Comp Endocrinol 2017; 244:164–177.
- 4 Guernsey MW, Chuong EB, Cornelis G, et al: Molecular conservation of marsupial and eutherian placentation and lactation. Elife 20172;6:e27450.
- 5 Runciman SI, Baudinette RV, Gannon BJ: Postnatal development of the lung parenchyma in a marsupial: the tammar wallaby. Anat Rec 1996;244:193–206.
- 6 Kwek JH, Iongh RD, Digby MR, et al: Cross- fostering of the tammar wallaby (Macropus eugenii) pouch young accelerates fore-stomach maturation. Mech Dev 2009;126:449–463.
- 7 Basden K, Cooper DW, Deane EM: Development of the lymphoid tissues of the tammar wallaby Macropus eugenii. Reprod Fertil Dev 1997;9:243–254.
- 8 Harrison PH, Porter M: Development of the brachial spinal cord in the marsupial Macro- pus eugenii (tammar wallaby). Dev Brain Res 1992;70:139–144.
- 9 Saunders NR, Adam E, Reader M, et al: Monodelphis domestica (grey short-tailed opossum): an accessible model for studies of early neocortical development. Anat Embryol 1999;180:227–236.
- 10 Tyndale-Biscoe CH, Janssens PA (eds): The Developing Marsupial: Models for Biomedical Research. Heidelberg, Springer, 1988.
- 11 Menzies BR, Shaw G, Fletcher TP, et al: Perturbed growth and development in marsupial young after reciprocal cross-fostering be- tween species. Reprod Fertil Dev 2007;19: 976–983.
- 12 Trott JF, Simpson KJ, Moyle RL, et al: Mater- nal regulation of milk composition, milk pro- duction, and pouch young development during lactation in the tammar wallaby (Macropus eugenii) Biol Reprod 2003;68:929–936.
- 13 Lefevre CM, Sharp JA, Nicholas KR: Evolution of lactation: ancient origin and extreme adaptations of the lactation system. Ann Rev Genomics Hum Genet 2010;11:219–238.
- 14 Wanyonyi SS, Lefevre CM, Sharp JA, et al: The extracellular matrix locally regulates asynchronous concurrent lactation in tam- mar wallaby (Macropus eugenii). Matrix Biol 2013;32:342–351.
- 15 Waite R, Giraud A, Old J, et al: Cross-fostering in Macropus eugenii leads to increased weight but not accelerated gastrointestinal maturation. J Exp Zoolog A Comp Exp Biol 2005;303:331–344.
- 16 Modepalli V, Hinds LA, Sharp JA, et al: Role of marsupial tammar wallaby milk in lung maturation of pouch young. BMC Dev Biol 2015;15:16.
- 17 Modepali VN, Hinds L, Lefevre CM, et al: Marsupial tammar wallaby delivers milk bio- actives to altricial pouch young to support lung development. Mech Dev 2016;142:22– 29.
- 18 Modepalli V, Kumar A, Hinds LA, et al: Differential temporal expression of milk miRNA during the lactation cycle of the marsupial tammar wallaby (Macropus eugenii). BMC Genomics 2014;15:1012.
- 19 Sharp JA, Lefevre CM, Watt A, et al: Analysis of human breast milk cells: gene expression profiles during pregnancy, lactation, involution, and mastitic infection. Funct Integr Genomics 2016;16:297–321.
- 20 Lee AC, Katz J, Blencowe H, et al: National and regional estimates of term and preterm babies born small for gestational age in 138 low-income and middle-income countries in 2010. Lancet Global Health 2013;1:e26– e36.
- 21 Svedenkrans J, Henckel E, Kowalski J, et al: Long-term impact of preterm birth on exercise capacity in healthy young men: a national population-based cohort study. PLoS One 2013;8:e80869.