Food Protein-Induced Enterocolitis Syndrome and Proctocolitis
Key Messages
- Food protein-induced enterocolitis syndrome (FPIES) is a non-IgE-mediated syndrome of food allergy in infancy.
- FPIES puts the child at risk of severe vomiting and dehydration, responding to intravenous fluids and not to adrenalin.
- Food protein-induced allergic proctocolitis is a common cause of rectal bleeding in the breastfed neonate.
Keywords
Food allergy · Non-IgE-mediated allergic reactions · Milk · Soy · Enterocolitis · Proctocolitis
Abstract
Non-IgE-mediated, also labeled cell-mediated, allergic reactions to foods are more common than usually thought and probably account for approximately more than 40% of cases of cow’s milk allergy during infancy and young childhood. Food allergy is now described in the form of syndromes, among which food protein-induced enterocolitis syndrome (FPIES) and food protein-induced allergic proctocolitis (FPI- AP) are gaining increased recognition. FPIES occurs in infancy but may also occur in older children and in adults. The dominant symptom is emesis, repetitive in the chronic FPIES form and explosive in the acute form. Acute FPIES begins 1– 4 h following ingestion of the offending food. Diarrhea is frequent, between 5 and 10 h later, and may be accompanied by lethargy and dehydration, which both characterize severity. Cow’s milk is the most frequent food trigger, followed by soy. FPIES may develop up to 1 year of age, but may also oc- cur in the newborn, and is possible in exclusively breastfed infants, in relation with the mother’s consumption of offending foods. FPIES may occur to solid foods (grains like rice or oat, meats, fish, egg, and vegetables). When starting during infancy, FPIES has a good prognosis and disappears grossly at 2 years of age. FPIES to fish or shellfish is more frequent in older children and adults and is long lasting. International consensus guidelines for the diagnosis and management of FPIES have been published recently. FPIAP starts in the first few months of life and is typically manifested with rectal bleeding in well-appearing breastfed infants during the first months of life in reaction to cow’s milk consumed by the mother. The condition is transient but represents one of the major causes of colitis during infancy.
Food Protein-Induced Enterocolitis Syndrome
Food protein-induced enterocolitis syndrome (FPIES) is a disease of infancy which may also occur in older children and in adults. The dominant symptom is emesis which appears repetitive in the chronic form of FPIES and explosive in the acute form. Acute FPIES be- gins 1–4 h following ingestion of the offending food, is followed by diarrhea, between 5 and 10 h later, and may be accompanied by lethargy and dehydration, which characterize severity [1]. There are neither respiratory nor skin manifestations. Cow’s milk is one of the most frequent food triggers, followed by soy. The syndrome may develop up to 1 year of age but may also occur in the newborn. FPIES to cow’s milk or soy is possible in exclusively breastfed infants, in relation with the mother’s consumption of offending foods. Delayed-onset FPIES is usually a consequence from delayed introduction of cow’s milk, soy, or solid foods, especially in breastfed infants. FPIES may occur to solid foods (grains like rice or oat, meats, fish, egg, and vegetables). When starting during infancy, FPIES has a good prognosis and disappears grossly at 2 years of age. FPIES to fish or shellfish is more frequent in older children and adults and is long lasting. International consensus guidelines for the diagnosis and management of FPIES have been published recently [2].
Clinical Presentation of FPIES
FPIES may appear either early in infants younger than 9 months or later. It may be mild-moderate or severe, and also classic, with no detectable food-specific IgE, or atypical when food-specific IgE are present [2].
The following description explains the chronic form of FPIES, when the offending food is consumed regularly, typically during infancy, and the acute form, usually associated with the accidental ingestion of the offending food, typically during an elimination diet. The acute form is also seen in older children or adults, when the offending food is not a staple food and is consumed only occasionally.
Chronic Form
In infants, the first period of the disease is marked by the chronic form, related with the permanent consumption of the offending food, typically cow’s milk proteins at this age. It usually resolves within 3–10 days of elimination diet, mainly with a hypoallergenic formula in infants with milk FPIES [3]. In severe cases, FPIES starts in the first days of life in infants fed cow’s milk- or soy-based formula. Intermittent emesis and chronic, sometimes bloody, diarrhea occurs without a specific temporal relationship with ingestion of the offending food [3–5]. Many associated symptoms may occur, such as abdominal dis- tension, dehydration, weight loss, and lethargy. Biology may show anemia, elevated white blood count with eosinophilia, and hypoalbuminemia and metabolic acidosis. Abdominal radiographs may show intramural gas, suggesting necrotizing enterocolitis, and/or leading to anti-biotherapy following sepsis evaluation [6, 7]. FPIES may also manifest as acidemia and transient methemoglo-binemia, especially in young infants with severe reactions, with some requiring a treatment, methylene blue and bicarbonate, according to cases [8].
Acute Form (Infancy)
Following a period of avoidance, the accidental or deliberate (during a food challenge) exposition to food (cow’s milk proteins, egg) induces acute symptoms. These occur within 1–4 h, mainly in the form of emesis, usually projectile and repetitive, sometimes up to more than 10 times. In the meantime, the child appears pale and lethargic. Diarrhea occurs in infants and in severe reactions and starts later on, from 5 to 10 h after food ingestion, sometimes bloody and containing mucous. A stool sample would evidence the presence of leukocytes, eosinophils, and increased carbohydrate [3]. In extreme cases, abdominal distension is severe enough to suggest ileus, wrongly leading to an exploratory laparotomy [9]. In contrast, diarrhea may lack in less severe acute reactions, for example during a food challenge (when a limited amount of offending food is given) as well as in old- er children [10] or adults, in whom emesis dominates. Hypotension is possible and may lead to hypovolemic shock [11, 12]. Blood samples during positive food challenges show increased blood neutrophil counts, with a peak at 6 h [3].
Acute Form (Adults)
In adults, FPIES always appears in the acute form, with a syndrome similar to the acute form in infants, with severe nausea, abdominal cramps, protracted vomiting, and diarrhea [11]. Responsible foods are mainly mollusks (scallop), crustacean shellfish, and fish.
Epidemiology and Responsible Foods
Few data relate to the epidemiology of FPIES. FPIES to cow’s milk was reported in 0.34% of infants under the age of 12 months in Israel [13]. In Australian children aged less than 24 months, the incidence of FPIES was 15.4/ 100,000/year [14].
Among delayed gastrointestinal immune reactions to cow’s milk, those with FPIES are considered to represent up to 40% in infants and young children with cow’s milk allergy. [15]. In a large American referral population [16], the most common foods identified were milk (67%), soy (41%), rice (19%), oat (16%), and egg (11%). However, in the Australian study [14], the most common FPIES trig- ger was rice (45%), followed by cow’s milk (33%) and egg (12%). In a retrospective Italian study (2004–2010), cow’s milk was the most common trigger food (65%), followed by fish, egg, rice, soy, corn, poultry, and goat’s milk [17]. FPIES may occur, rarely, in breastfed infants [18].
Most of the time (60%), patients react to a single food [16, 10], mainly cow’s milk and soy, with 40% of infants potentially reacting to both. Solid foods may also induce FPIES (rice, oat, barley, chicken, turkey, egg white, green pea, and peanut) [1]. Solid-food FPIES tends to start later than cow’s milk and soy FPIES, perhaps because solids are introduced later at around 6 months of age [10]. In an Italian study, the chief peculiarities of acute fish and shellfish FPIES [19], compared to more frequent cow’s milk or soy FPIES, were an older age of onset, longer persistence, possibility of tolerating fish other than the offending fish, and adverse reactions to shellfish.
Multiple FPIES is more common than usually thought. In the Italian study [17], 85% of children reacted to a single food. In the Australian study [14], 20% had 2 food triggers and 12% had ≥3 food triggers. Infants with FPIES to multiple versus single food groups were younger at the initial episode (4.6 vs. 5.8 months) and more frequently had FPIES to fruits, vegetables, or both (66% vs. 21%). Sixty-four percent of infants with FPIES to multiple foods, which included cow’s milk, had co-associated FPIES to solid foods. Infants with FPIES to fish reacted to other food groups in 42% of cases.
A family history of atopy is found in 40–80% of pa- tients, with a positive food allergy family history in 20% of the cases [7]. Atopic diseases may develop later in life in infants with FPIES, eczema (23–57%), asthma or rhinitis (20%), or drug hypersensitivity [7]. Importantly, IgE positivity to other foods may reach 40% [10, 16], suggesting a role for these antibodies in the pathophysiology of the disease, at least in some cases.
Pathophysiology
The mechanisms underlying FPIES remain poorly characterized [20, 21]. During acute FPIES, blood testing will reveal an elevated white blood cell count with neutrophilia and thrombocytosis. If the syndrome is severe, the patient may also exhibit metabolic acidosis and methemoglobinemia. Increased serum cortisol levels have been described on oral food challenge (OFC) in infants with FPIES [22]. Stools may be positive for leukocytes, eosinophils, and increased carbohydrate content.
When diagnostic criteria were not available, endoscopy was carried out in symptomatic infants with cow’s milk and/or soy FPIES, showing rectal ulceration and bleeding with friable mucosa [4]. When infants had FPIES manifested by chronic diarrhea, rectal bleeding, and/or failure to thrive, the main findings in radiographs were excess luminal fluid with air fluid levels, all signs which disappeared during appropriate elimination diet.
Natural History
Except for FPIES to fish and shellfish reported in older children and adults, FPIES develops during infancy and not beyond 1 year of age, suggesting a “window of physi- ologic susceptibility” [20, 23, 24].
FPIES is a condition which appears self-limiting and resolves without long-lasting sequelae [20]. Studies have been carried out in different countries and show great variations [25]. In Israel, cow’s milk FPIES resolved by 3 years of age in 90% of cases [13]. In Korea, resolution was observed by 2 years of age and for soy FPIES by 14 months of age [26]. In the Italian study, 48% achieved tolerance at a mean age of 29 months, and the age of achieved tolerance for cow’s milk was significantly lower compared to that of other foods (24 ± 8 vs. 53 ± 17 months) [17]. In the United States study, resolution of FPIES exhibited lower rates: 35% by 2 years of age, 70% by 3 years, and 85% by 5 years [16]. In Japanese patients with FPIES caused by cow’s milk, the rate of tolerance acquisition was 18.8, 56.3, 87.5, and 96.9% at the ages of 6, 12, 24, and 36 months, respectively [27].
Solid FPIES resolves later, and grossly 50% of children outgrow rice or oat FPIES by 4–5 years of age [10, 16, 25]. The potential resolution of FPIES to seafood in older children and adults is unknown.

Diagnosis of FPIES
History-taking is key for the diagnosis of FPIES, analyzing the clinical features, excluding other etiologies, and preparing the food challenge [28].
Very frequently, FPIES is not recognized at the first visit, whether in its chronic or acute form; owing to both the lack of associated typical cutaneous and respiratory allergic symptoms [28] and the lack of knowledge of this “emerging” syndrome among physicians [29–32]. This lack of knowledge includes the common belief that rice, oat, and vegetables are hypoallergenic and can never induce an allergic reaction.
Such as for many situations in food allergy, the stan- dard for diagnosing FPIES is the OFC. It is not mandatory when the clinical history shows repeated, frequently severe, reactions which disappear completely when the suspected food has been eliminated. Most of the time, OFCs are performed in order to check whether FPIES resolved and if the offending food may be reintroduced.
There is no biological test for FPIES such as for non-IgE-mediated gastrointestinal food allergies [33]. The presence of cow’s milk-specific IgE has been described in one-third of patients with milk FPIES [34], a figure that seems higher in Japan [35]. In patients with food-specific IgE antibodies after the diagnosis of FPIES or during the course of the disease, the latter appeared to be more protracted [18, 36]. Measurement of serum food-specific IgE levels thus seems useful to identify patients at risk for persistent FPIES. Skin prick tests will be negative.
The atopy patch test has been tested in several studies of FPIES, with conflicting results [16, 37, 38], thus it is not recommended in routine practice.
Diagnostic criteria that have been published are indi- cated in Table 1. Differential diagnosis is presented in Table 2.
Management of FPIES
Food avoidance is the mainstay of treatment, with spe- cific guidance for the treatment of accidental reactions and periodic reevaluations for tolerance, based mainly on OFC.
Avoidance
The elimination diet is similar to that done in food allergy. Cow’s milk and soy FPIES are the most frequent and require an appropriate feeding. Breastmilk is the best, as usual. Formula feeding relies mainly on extensively hydrolyzed milk protein formula or on amino-acid formulas, which are requested by one half of patients with milk FPIES [39]. In case of chronic cow’s milk and/or soy FPIES, symptoms resolve rapidly, within 1 week, often within 3 days of starting the elimination diet. Tube feeding or intravenous fluids may sometimes seem necessary at the beginning of treatment.

Oral Food Challenges
OFCs appear necessary, whether it is to establish the diagnosis of FPIES or to check the spontaneous evolution of the disease FPIES. The current recommendation is to challenge children every 18–24 months in patients without recent reactions. The exact timing of these OFCs to determine resolution has not been extensively investigated. The common attitude is an OFC within 12–18 months following the most recent reaction [23]. In Korea, a prospective study suggested earlier rechallenging: cow’s milk FPIES resolved in all patients by the age of 2 years and soy FPIES by the age of 14 months [26]. In Israel, cow’s milk FPIES was diagnosed in all children with milk by the age of 6 months, 50% resolved within the first year of life, 89% by the age of 2 years, and 90% by the age of 3 years [13].
Physician supervision for these OFCs is mandatory. A placement of a secure peripheral venous line is advisable in patients with previous severe reactions and in infants or older patients with anticipated difficult intravenous access: intravenous fluids and/or steroids are the most frequently used [17].
Treatment of Acute Reaction: A Need for Anticipation
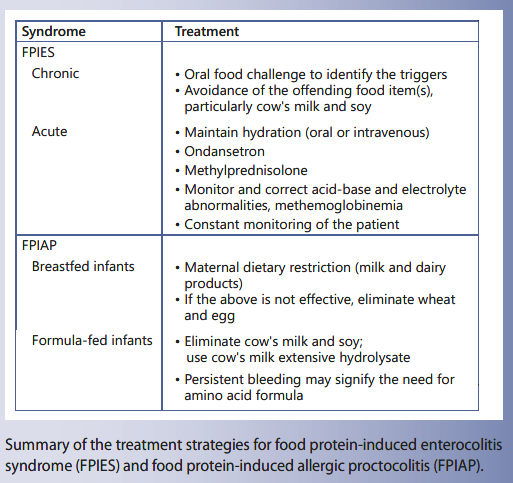
Guidelines for the treatment of acute FPIES have been recently published [2] and are summarized in Table 3.
The first-line therapy for severe acute reactions, whether they occur accidently or during an OFC, is rapid intravenous hydration, using 20 mL/kg normal saline bolus. The role of intravenous access during OFCs has been studied [40].
Intravenous steroids may be used in case of severe reactions, likely to reduce intestinal inflammation. Epinephrine is not the primary treatment but may be used following rehydration in case of severe hypotension and cardiovascular shock. Epinephrine does not improve emesis and lethargy, the latter responding much more to intravenous fluid administration.
Intravenous ondansetron seems effective for stopping emesis during an OFC for FPIES [41]. Ondansetron was given at a dosage of 0.2 mg/kg per dose together with intravenous physiologic saline bolus in 5 children above 3 years of age with emesis during OFC: emesis and lethargy resolved within 10–15 min in 4 children treated with in- travenous ondansetron and 1 child required an additional dose of ondansetron. In another child, ondansetron was given orally, and severe abdominal pain improved only with an additional intravenous ondansetron dose. In a small case series in young children, intramuscular on- dansetron was effective to manage acute FPIES during OFC carried out in the physician’s office [42].
All patients with FPIES need to be equipped with an emergency treatment plan, detailing the clinical features and the management of acute reactions. In case of a mild reaction, careful oral rehydration may be performed at home, whereas in case of a severe reaction, resuscitation necessitates the emergency department or inpatient unit.
Summary for FPIES
FPIES is gaining more and more interest and recognition, with its frequency potentially increasing. The syndrome is dangerous in its acute form and its recognition by health care professionals should be encouraged. The pathophysiology is still missing and its occurrence in both infants and adults remains unexplained. Guidelines now considerably help standardizing the treatment and providing families a guide to handle this very specific form of food allergy.
Food Protein-Induced Allergic Proctocolitis
Food protein-induced allergic proctocolitis (FPIAP) starts in the first few months of life. It was first described by Lake et al. [43] in 1982 in 6 breastfed infants with rectal bleeding that appeared during the first month of life. Well-appearing infants have blood-streaked stools, indicating a benign and transient condition, but probably one of the major causes of colitis during infancy [44–46].
Clinical Features
FPIAP frequently occurs in breastfed infants where it is usually caused by cow’s milk, soy, egg, and corn proteins, whereas in formula-fed infants, FPIAP is typically caused by cow’s milk and soy proteins.
The disease usually presents within the first 6 months of life, usually within the first month with normal to moderately loose stools and intermittent blood streaks. The onset is usually insidious, and a more or less prolonged interval separates the introduction of the food protein and the appearance of symptoms.
FPIAP is common in breastfed infants, accounting for as many as 60% of cases [47]. The exact prevalence is unknown, but in infants with rectal bleeding, FPIAP might account for 18–64% of cases [48, 49]. In breastfed infants, the disease usually develops later and less severely on histologic analysis [43, 47, 49]. A gradual resolution of symptoms occurs with elimination of the offending food from the mother’s diet, allowing the mother to go on with breastfeeding [47].
Bleeding may persist in breastfed infants, despite maternal avoidance of food(s), probably in relation with the difficulty in removing all sources of allergen from the diet or in identifying all the responsible allergens. In these cases, a milk protein hydrolysate formula, or an amino acid- based formula, may be necessary and usually resolves bleeding, typically within 48–72 h.
The disease is typically limited but may be accompanied by colic or increased frequency of bowel movements, and sometimes increased gas (up to 30% of patients), intermittent emesis (up to 27%), pain on defecation (22%), or abdominal pain (up to 20%) may be present [1]. How- ever, the infants typically appear well and there is no failure to thrive.
A positive family history of atopy, elevated serum IgE levels, and peripheral blood eosinophilia are sometimes seen. In stools, smears of mucus may show increased polymorphonuclear neutrophils. Mild anemia or hypoal-buminemia may develop. In the series of Lake [47], 6 of 21 patients developed iron deficiency anemia despite iron supplementation, but the weight gain was normal and the disease had disappeared within 1 year of age.
Tolerance of the offending food occurs by 1–3 years of age with the majority by 1 year. Spontaneous resolution of bleeding occurs in up to 20% of breastfed infants with- out changes in the maternal diet [50]. In contrast, when the maternal elimination diet seems not efficient, persistence of rectal bleeding does not hamper an excellent long-term prognosis.
Pathophysiology
FPIAP predominantly affects the rectosigmoid and the disease is typically associated with breastfeeding, meaning that the children react at the distal part of the intestine to very little amounts of the offending food. At endoscopy, lymphoid nodular hyperplasia is associated with focal erythema surrounding the lymph nodes.
Diagnosis
Diagnosis exclusively relies on the clinical history, with rectal bleeding disappearing following elimination diet, in the mother or the child, within usually 72–96 h [47], sometimes much later, underlining the need for elimination diets of at least 2–4 weeks. Persistence of occult blood is possible [48] and may suggest allergy to other unrecognized foods. An allergy workup is still necessary: even though tests for IgE-mediated food hypersensitivity are mostly inconsistent, it remains important to detect IgE-mediated milk allergies.
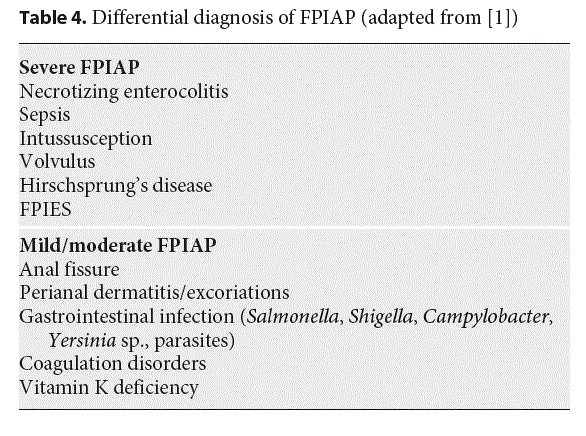
Exclusion of other causes of rectal bleeding, such as infection, necrotizing enterocolitis, intussusception, or anal fissure, is important (Table 4). Campylobacter infection is a differential diagnosis, especially since symptoms may be mild at this age, with only rectal bleeding [51]. In case of persistent bleeding, ultrasonography eliminates any anatomic abnormalities or intussusception. Importantly, the bleeding is often attributed to perirectal fissures. Typically, fissures accompanying constipation tend to present with streaks of blood on hard, formed stool, at the opposite of frothy, mucousy stools of FPIAP. However, anal fissures may be a symptom of cow’s milk allergy [52–54] and thus associated with the mucosal lesions of FPIAP, which means that their presence does not rule out FPIAP and should be another argument to begin the elimination diet. There are no reports of inflammatory bowel disease in infants with FPIAP followed for more than 10 years, but inflammatory bowel disease may, rarely, begin during the first months of life.
Management
Treatment is dietary restriction in the mother when the child is breastfed and in the child when he or she is formula fed. Elimination is first focused on milk and dairy products. Soy formula may induce bleeding in a subset of infants reacting to cow’s milk, so that it is better to eliminate also soy at least for the diagnosis period of the elimination diet. In the breastfed infants, when bleeding is not controlled by the elimination of cow’s milk and soy, additional eliminations may be considered including wheat and egg. When the child is not breastfed or when the mother decides to stop breastfeeding, a milk hydrolysate may be sufficient, but the persistence of bleeding underlines the need for an amino-acid formula. Recurrence of bleeding is common when rechallenge takes place within the first 6 months. Usually, skin prick tests and the detection of food-specific IgE are negative, and food introduction takes place at home, with a gradual increase over 2 weeks.
Summary for FPIAP
FPIAP is a common condition of infancy. The prognosis is good with a majority of cases resolving in the first year of life. Cow’s milk is the major offending food. Treatment relies on milk elimination, either in the mother of a breastfed infant or in the child. Accidental exposure usually triggers limited damages, e.g., relapse of rectal bleeding.
Disclosure Statement
C. Dupont received honoraria for the writing of this article, is a member of a Nestlé scientific advisory board and is co-founder of DBV Technologies, a company which shares a joint venture with Nestlé.
The writing of this article was supported by Nestlé Nutrition Institute.
References
- 1 Nowak-Węgrzyn A. Food protein-induced enterocolitis syndrome and allergic proctocolitis. Allergy Asthma Proc. 2015 May-Jun; 36(3):172–84.
- 2 Nowak-Węgrzyn A, Chehade M, Groetch ME, Spergel JM, Wood RA, Allen K, et al. In- ternational consensus guidelines for the diagnosis and management of food protein-in- duced enterocolitis syndrome: Executive summary-Workgroup Report of the Adverse Reactions to Foods Committee, American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol. 2017 Apr;139(4): 1111–1126.e4.
- 3 Powell GK. Milk- and soy-induced enterocolitis of infancy. Clinical features and standardization of challenge. J Pediatr. 1978 Oct;93(4): 553–60.
- 4 Gryboski JD. Gastrointestinal milk allergy in infants. Pediatrics. 1967Sep;40(3):354–62.
- 5 Nomura I, Morita H, Hosokawa S, Hoshina H, Fukuie T, Watanabe M, et al. Four distinct subtypes of non-IgE-mediated gastrointestinal food allergies in neonates and infants, distinguished by their initial symptoms. J Allergy Clin Immunol. 2011 Mar;127(3):685–8.e1.
- 6 Mehr S, Kakakios A, Frith K, Kemp AS. Food protein-induced enterocolitis syndrome: 16- year experience. Pediatrics. 2009 Mar;123(3): e459–64.
- 7 Nowak-Węgrzyn A, Sampson HA, Wood RA, Sicherer SH. Food protein-induced enterocolitis syndrome caused by solid food proteins. Pediatrics. 2003 Apr;111(4 Pt 1):829–35.
- 8 Murray KF, Christie DL. Dietary protein in- tolerance in infants with transient methemoglobinemia and diarrhea. J Pediatr. 1993 Jan; 122(1):90–2.
- 9 Jayasooriya S, Fox AT, Murch SH. Do not laparotomize food-protein-induced enterocolitis syndrome. Pediatr Emerg Care. 2007 Mar; 23(3):173–5.
- 10 Caubet JC, Ford LS, Sickles L, Järvinen KM, Sicherer SH, Sampson HA, et al. Clinical features and resolution of food protein-induced enterocolitis syndrome: 10-year experience. J Allergy Clin Immunol. 2014 Aug;134(2): 382–9.
- 11 Fernandes BN, Boyle RJ, Gore C, Simpson A, Custovic A. Food protein-induced enterocolitis syndrome can occur in adults. J Allergy Clin Immunol. 2012 Nov;130(5):1199–200.
- 12 Coates RW, Weaver KR, Lloyd R, Ceccacci N, Greenberg MR. Food protein-induced enterocolitis syndrome as a cause for infant hypotension. West J Emerg Med. 2011 Nov; 12(4):512–4.
- 13 Katz Y, Goldberg MR, Rajuan N, Cohen A, Leshno M. The prevalence and natural course of food protein-induced enterocolitis syndrome to cow’s milk: a large-scale, prospective population-based study. J Allergy Clin Immunol.2011 Mar;127(3):647–53.e1.
- 14 Mehr S, Frith K, Barnes EH, Campbell DE, Allen K, Barnes E, et al.; FPIES Study Group. Food protein-induced enterocolitis syndrome in Australia: A population-based study, 2012-2014. J Allergy Clin Immunol. 2017 Nov;140(5):1323–30.
- 15 Sicherer SH. Food protein-induced enterocolitis syndrome: case presentations and management lessons. J Allergy Clin Immunol. 2005 Jan;115(1):149–56.
- 16 Ruffner MA, Ruymann K, Barni S, Cianferoni A, Brown-Whitehorn T, Spergel JM. Food protein-induced enterocolitis syndrome: insights from review of a large referral population. J Allergy Clin Immunol Pract. 2013 Jul- Aug;1(4):343–9.
- 17 Sopo SM, Giorgio V, Dello Iacono I, Novembre E, Mori F, Onesimo R. A multicentre retrospective study of 66 Italian children with food protein-induced enterocolitis syndrome: different management for different phenotypes. Clin Exp Allergy. 2012 Aug;42(8):1257–65.
- 18 Tan J, Campbell D, Mehr S. Food protein-induced enterocolitis syndrome in an exclusively breast-fed infant-an uncommon entity. J Allergy Clin Immunol. 2012;129:873, author reply 873–4.
- 19 Miceli Sopo S, Monaco S, Badina L, Barni S, Longo G, Novembre E, et al. Food protein- induced enterocolitis syndrome caused by fish and/or shellfish in Italy. Pediatr Allergy Immunol. 2015 Dec;26(8):731–6.
- 20 Caubet JC, Nowak-Węgrzyn A. Current understanding of the immune mechanisms of food protein-induced enterocolitis syndrome. Expert Rev Clin Immunol. 2011 May; 7(3):317–27.
- 21 Berin MC. Immunopathophysiology of food protein-induced enterocolitis syndrome. J Allergy Clin Immunol. 2015 May;135(5):1108– 13.