Food Allergy Prevention and Treatment by Targeted Nutrition
Key Messages
- In view of the dramatic rise in the prevalence of food allergy globally, effective prevention strategies have become a public health priority.
-
The early introduction of complementary diet and food allergens from 4 months of age is currently one of the most promising approaches in the prevention of food allergies.
- While the strict food allergen avoidance remains the main treatment strategy for food allergies, immunotherapy via the oral or epicutaneous route have emerged as effective and feasible future treatment strategies.
Keywords
Allergy · Atopy · Breastfeeding · Infant formula · Microbiome · Prevention
Abstract
In view of the dramatic rise in the prevalence of food allergy globally, effective prevention strategies have become a public health priority. Several models have emerged around the etiology of food allergy, including the hygiene hypothesis, dual allergen exposure hypothesis, and vitamin D hypothesis. These form the basis for current and potential prevention strategies. Breastfeeding remains a key pillar of primary allergy prevention. Other nutritional interventions, including the use of whey-based, partially hydrolyzed formula in non- breastfed infants, also play an important role. In recent years, there has been a shift away from prolonged food allergen avoidance to the proactive allergen introduction from 4 months of age. This approach is supported by 2 pivotal randomized clinical trials showing that the early introduction of peanut and other food allergens significantly reduces the risk of food allergy. However, the implementation of this strategy at the population level still raises significant logistic problems, including patient selection and development of suitable food formats for young infants. Other prevention strategies, including vitamin D supplementation, are currently under evaluation. Maternal elimination diets during pregnancy and lactation are not recommended for allergy prevention. The treatment of food allergies has also seen major transformations. While strict allergen avoidance is still the key treatment principle, there is a greater focus on desensitization and tolerance induction by oral and epicutaneous immunotherapy. In addition, specialized hypoallergenic infant formulas for the treatment of infants with cow’s milk allergy have undergone reformulation, including the addition of lactose and probiotics in order to modulate the gut microbiome and early immune responses. Further research is needed to inform the most effective food allergy prevention strategies at the population level. In addition, the wider application of food allergen immunotherapy may provide better health outcomes and improved quality of life for families affected by food allergies.
Introduction
Over the past 2 decades, the prevalence of allergic disorders has increased dramatically [1–3]. The greatest increase has been observed in infants and children with food allergies or atopic eczema [4, 5]. In Europe and Northern America, food allergy is estimated to occur in 1–5% of the population [1]. In Australia, a population-based study found a prevalence of challenge-proven food allergy of over 10% which is the highest rate globally [6]. Overall, prevalence figures for food allergy and anaphylaxis appear to be steadily rising [7]. Effective allergy prevention has therefore become a global public health priority [8].
Nutritional interventions play a central role in the prevention and treatment of food allergies (Table 1). In recent years, clinical approaches have undergone significant changes [3]. In the food allergy prevention space, greater focus has been placed on the early introduction of the complementary diet in infancy [9] (Fig. 1). This is in contrast to the previous approach of prolonged food allergen avoidance which, in hindsight, may have paradoxically increased the rate of food allergies [10–12]. In the area of food allergy treatment, there have also been major advances, including a shift from mere food allergen avoidance to proactive food allergen immunotherapy [13]. In addition, there is renewed interest in the role of gut microbiome-modifying therapies in an attempt to promote immunological tolerance development via the gut-associated immune system [14–16]. This review summarizes the previous and current approaches to dietary food allergy prevention and treatment and highlights areas of uncertainty or controversy, as well as priorities for future research.

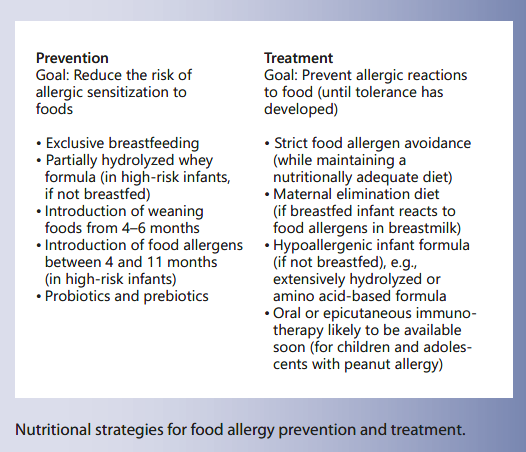
Table 1. Nutritional strategies for the prevention of food allergy
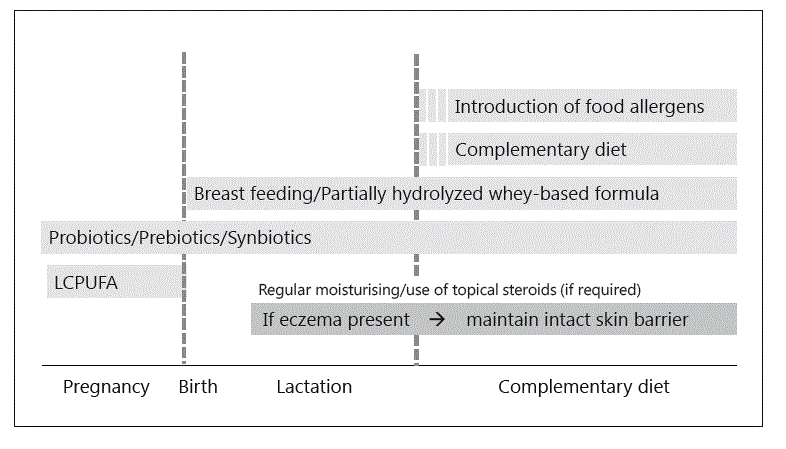
Fig. 1. Integrated model of nutritional interventions and skin care for food allergy prevention.
Breastfeeding
Breastfeeding is one of the main pillars in both food allergy prevention and treatment [17–21]. Breast milk provides the most appropriate source of nutrition for the young infant as it contains a specific nutrient mixture, growth factors, and protective maternal antibodies. The World Health Organization (WHO) guidelines on complementary feeding of 2001 recommend exclusive breast- feeding for at least 6 months. However, this recommendation has been challenged in countries with a high prevalence of food allergies, as the early dietary introduction of allergens appears to protect from food allergies [22, 23]. Recent guidelines on the prevention of food allergies from Europe, the USA, and Australia have recommended the introduction of solids from 4–6 months of age [24– 28].
Breastfeeding is associated with the establishment of fecal microbiota high in Bifidobacteria [29]. Human milk oligosaccharides (HMO) promote the colonization of the gut with Bifidobacteria which is thought to promote mucosal tolerance via interaction with regulatory T-lymphocytes and Toll-like receptors [30]. Breastfeeding itself does not appear to confer a strong protective effect against food allergies [21]. However, the duration of exclusive breastfeeding appears to influence the risk of allergic disease [31, 32]. The protective effect of breastfeeding on eczema in the first 2 years of life appears to be modified by maternal allergy status [33]. Exclusively breastfed infants can express clinical manifestations of food allergy, including food protein-induced proctocolitis and multiple food intolerance of infancy [34–37]. These often respond to treatment with hypoallergenic maternal elimination diets which eliminate cow’s milk or other food allergens [38, 39]. In some infants who failed a trial of maternal dietary elimination, treatment with a hypoallergenic formula may be required [40–42]. Maternal elimination diets during pregnancy and lactation for the purpose of allergy prevention are not recommended [18, 43].
Prevention
Primary food allergy prevention aims to reduce the infant’s risk of sensitization to food allergens [44]. By contrast, secondary prevention aims to prevent the clinical expression of allergic disease in individuals who are either allergen sensitized or who already manifest other allergic disorders, such as atopic dermatitis or asthma. The prevention of food allergies and atopic dermatitis by nutritional interventions has been explored for the past 2 decades with a broad range of approaches. In addition to the promotion of breastfeeding, these have included the use of partially hydrolyzed formula (PHF) and a range of maternal elimination diets [43, 45]. Supplementation with probiotics, prebiotics, and specific nutrients has also been explored [46, 47]. Some of these interventions have been trialed in high-risk populations, either in families with a history of allergies, or in infants who are showing evidence of food sensitization or eczema. Other studies have assessed the preventive effect of interventions at the population level without selecting for allergic history. This distinction is important when the findings of prevention trials are translated into population-based health policies [22, 48].
Prevention strategies have been developed around 3 main hypotheses on the etiology of food allergies: the hygiene hypothesis, the dual allergen exposure hypothesis, and the vitamin D hypothesis [11, 49, 50]. The following sections will summarize current preventive strategies in the context of these hypotheses.
Hygiene Hypothesis
Gut microbiota and environmental microbial burden play a central role in early immune development and are likely to influence immunological events that lead to allergy [49, 51, 52]. The hygiene hypothesis assumes that there is an immune deviation to T-helper 2 reactions due to reduced early microbial exposure and a lack of fecal microbial diversity [53, 54]. For example, growing up in a rural farm environment has been shown to significantly reduce the risk of asthma and allergic disease in children [49, 55]. There are significant differences in the gut microbiota profiles between allergic and nonallergic infants and children [56, 57]. Infants with IgE-associated eczema have significantly reduced fecal microbial diversity in the first month of life, compared to nonatopic infants [54, 58]. Modification of early gut colonization and fecal microbial diversity in infancy may thus provide an avenue for preventive or therapeutic strategies [59]. Probiotic or prebiotic supplementation has been shown to modify the risk of allergies, particularly for atopic dermatitis in infancy [60–62]. The World Allergy Organization Guidelines recommend the use of probiotics and prebiotics for the prevention of eczema and allergies, but caution that the available evidence is of very low certainty [63, 64].
Probiotics
Infants with allergies have been shown to have significantly lower numbers of fecal Bifidobacteria, compared to healthy infants [65]. Allergy prevention via supplementation with probiotic bacteria therefore appears to be a promising approach. The effects of probiotics are mainly mediated via the innate immune system (Toll-like receptors), resulting in the promotion of T-helper 1 differentiation, production of regulatory cytokines (IL-10 and TGF-beta) and enhanced intestinal IgA responses [66]. Several studies have demonstrated that perinatal administration of probiotics to mothers in the last weeks of pregnancy and to infants in the first few months of life was associated with a significant reduction in atopic eczema [67–69]. Nevertheless, results have been varied, depending on the probiotic strain, dose, timing and food matrix used. A study using Lactobacillus acidophilus (LAVRI A1) even showed a paradoxical increase in allergic sensitization [70]. These studies highlight that clinical outcomes depend on the specific probiotic strains used. The role of probiotics in allergy prevention requires further study [46].
Prebiotics
HMO are complex, nondigestible oligosaccharides with prebiotic properties in breast milk which provide a specialized substrate for Bifidobacteria. In the past, infant formulas were devoid of prebiotic oligosaccharides [71]. Over the past decade, several manufactured prebiotics have been added to infant formula, including plant-based long-chain fructo-oligosaccharides (FOS) and short- chain galacto-oligosaccharides (GOS). GOS and FOS have been shown to increase counts of fecal Bifidobacteria in formula-fed infants [72, 73]. A randomized study examined the effects of a FOS/GOS-supplemented hydrolyzed formula on atopic eczema in formula-fed infants during the first 6 months of life [74]. In that study, the FOS/GOS group had significantly lower rates of eczema compared to the placebo group, although eczema severity was similar for both treatment arms. A more recent European multi-center randomized controlled trial assessed the effect of prebiotics in healthy, low-risk infants from 8 weeks to 12 months [75]. Prebiotics reduced the incidence of atopic dermatitis by 44% at 12 months. Again, disease severity was not affected. Further studies are needed to assess the role of GOS and FOS in allergy prevention [62].
HMO in breast milk provide the substrate for specific microbes and significantly influence early microbial gut colonization [76, 77]. Recently, 2 manufactured HMO (2′-fucosyllactose and lacto-N-neotetraose) have been added to standard cow’s milk-based formula [78]. In pre-clinical studies, HMO have been shown to attenuate allergic responses in cow’s milk-sensitized mice [79]. The role of HMO in the prevention and treatment of food allergies is at this stage not clearly defined but represents a promising area for future research [80, 81].
Dual Allergen Exposure Hypothesis
The dual allergen exposure hypothesis (via skin and gut) is based on the observation that infants with eczema have a high risk of developing IgE-mediated food allergies [11]. While allergen contact via eczematous skin may cause allergic sensitization, the exposure via the gastrointestinal tract is more likely to induce immunological tolerance [11, 82, 83]. Prolonged avoidance of a food allergen in infants with eczema may paradoxically increase the risk of food allergies [12, 84]. Previously, the delayed introduction of common food allergens (cow’s milk after 12 months, egg after 2 years, and peanut after 3 years) was recommended in an attempt to prevent food allergy [10]. Findings from several small studies provided support for the concept of a “window period” for tolerance induction, whereby tolerance is more likely to be achieved if weaning solids are introduced between 4 and 6 months of age. This reflects feeding practices in many European countries, but is not supported by the WHO guidelines on complementary feeding.
The Australian HealthNuts Study showed that the risk of developing egg allergy increased significantly if egg was introduced after 12 months of age [85]. This finding prompted to question the recommendation of delaying the introduction of egg beyond 12 months of age. The LEAP (Learning Early about Peanut) study was the pivotal study demonstrating that the early introduction of pea- nut into the infants diet from 4 months conferred a protective effect against peanut allergy in high-risk infants [86]. This study was based on the observation that infants in Israel who were exposed to peanut in a teething snack had a low risk of peanut allergy, while Jewish infants in the United Kingdom who introduced peanut generally after 12 months of age had a high risk. The subsequent clinical study enrolled infants with pre-existing egg allergy or eczema and randomized them to introduce peanut from 4 months, or to continue strict peanut avoidance. The study showed overwhelming evidence of a protective effect against peanut allergy (70– 86% relative incidence reduction) in those who introduced peanut early between 4 and 11 months of age. A supplementary analysis found that the skin prick test wheal diameter at the time of peanut introduction predicted the tolerance development in those who avoided peanut, with the greatest benefit seen between 6 and 11 months [87]. This analysis provided additional insights on the best timing of the dietary introduction of food allergens in high-risk infants.
A second study, the Enquiring about Tolerance (EAT) study, prospectively examined if the early introduction of 6 food allergens (from 4 months of age) while breastfeeding could reduce the risk of food allergy in a nonallergic population [88]. On per-protocol analysis, there was a significant protective effect against food allergy. However, the study overall failed on intention-to-treat analysis due to a large proportion of participants who were unable to adhere to the study regimen. This raised questions around the logistics of introducing foods early in infancy, including finding suitable food formats that would allow the delivery of food proteins in adequate doses to breast- fed infants [22, 27].
Partially Hydrolyzed Formula
The role of hydrolyzed formula in allergy prevention has been studied for more than 2 decades. Several studies have explored the tolerogenic potential of cow’s milk peptides in hydrolyzed formula. The German Infant Nutritional Intervention (GINI) study is to date the largest, quasi-randomized trial examining the role of hydrolyzed formula in the prevention of allergies [89]. Infants with a family history of allergies were randomized to receive cow’s milk-based formula, whey-based PHF, whey-based extensively hydrolyzed formula (EHF), or casein-based EHF at the time of weaning. That study found a sustained protective effect against atopic eczema for whey-based PHF and casein-based EHF [89]. Follow-up studies of the GINI cohort have demonstrated a sustained preventive effect of hydrolyzed formulas until 10 years of age, compared to cow’s milk-based formula [90]. A Cochrane review on hydrolyzed formulas in allergy prevention found a limited beneficial effect, compared to cow’s milk-based formula, in “high-risk” in- fants with a family history of atopy [91]. Two other meta- analyses also confirmed a preventive effect, mainly for atopic dermatitis [92, 93]. Others have questioned the role of PHF and cautioned against overstating its preventive effects [94, 95]. Boyle et al. [96] in their meta-analysis found no support for a preventive effect of PHF against allergic disease. However, pooling of data on hydrolyzed formulas in meta-analyses may be problematic due to significant heterogeneity of PHF products. A more recent meta-analysis addressed this issue and only included studies using 100%-whey PHF [97]. That study found a preventive effect for all allergies and eczema, but acknowledged limitations in the certainty of available data. The current Allergy Prevention Guidelines by the European Academy of Allergy and Clinical Immunology (EAACI) recommend the use of PHF with a documented preventive effect in infants at high-risk of allergy if breastfeeding is insufficient or not possible [98].
Vitamin D Hypothesis
Several studies have demonstrated an association between low vitamin D levels and food allergy [99, 100]. An Australian study showed that vitamin D insufficiency (serum level <50 nmol/L) was associated with a significantly increased risk of egg and/or peanut allergy [101]. This finding concurred with the observation that the prevalence of food allergy and eczema follows a north-south gradient, being more common in regions with less sun exposure and lower skin-derived vitamin D levels [102]. Adequate vitamin D levels in the first year of life may therefore provide protection against the development of food allergies. By contrast, vitamin D may also have undesirable immune-modulating effects and, in high doses, increase the risk of allergic sensitization. Vitamin D has been shown to inhibit the maturation of dendritic cells and impede the development of T-helper 1 responses. In theory, vitamin D therefore could increase the risk of allergic disorders in infancy [103]. This is supported by a recent German birth cohort study (LINA study) which found that high vitamin D levels during pregnancy and at birth were associated with an increased risk of food allergy [104]. The varying effects of vitamin D on allergy risk have been explained by a U-shaped dose response curve, i.e., normal vitamin D levels may confer a protective effect while abnormally high or low levels may increase the allergy risk [100]. The aforementioned studies suggest that both vitamin D insufficiency and oversupplementation are risk factors for allergies [99]. The VITALITY trial, a prospective randomized trial, is currently underway to assess the role of postnatal vitamin D supplementation as a preventive strategy against IgE-mediated food allergy, eczema, and lower respiratory tract infections [105].
Omega-3 Long-Chain Polyunsaturated Fatty Acids
Maternal diets high in omega-3 long-chain polyunsaturated fatty acids (LCPUFA) are thought to have a protective effect against the development of allergies in the newborn [106]. Supplementation with docosahexaenoic acid and eicosapentaenoic acid during pregnancy has been shown to increase LCPUFA concentrations in breast milk [107]. A large randomized clinical trial of maternal fish oil supplementation during pregnancy demonstrated a significant decrease in cord blood concentrations of Th-2 cytokines (IL-4 and IL-13) as well as increased levels of oral tolerance-inducing TGF-beta [108]. Palmer et al. [109]assessed the effect of high-dose fish oil supplementation in high-risk infants (with a positive family history of atopy). Pregnant mothers were randomized to receive either 800 mg docosahexaenoic acid plus 100 mg eicosapentaenoic acid or vegetable oil from 21 weeks’ gestation until delivery. Primary outcomes were infantile eczema and food sensitization at 12 months of age. Infants in the fish oil-supplemented group had significantly lower rates of atopic eczema and egg sensitization. In another study by the same group [110], high-risk infants were randomized to 280 mg docosahexaenoic acid plus 110 mg eicosapentaenoic acid daily or olive oil (control) from birth to 6 months of age. In that study, between-group comparisons revealed no differences in allergic sensitization, eczema, asthma, or food allergy. In summary, fish oil supplementation during pregnancy reduced the risk of atopic eczema and food sensitization, whereas dietary supplementation after birth appeared to be ineffective.
Treatment
The treatment of food allergies relies on the strict elimination of the offending allergens. In exclusively breastfed infants who react to allergens via breast milk, maternal elimination diets have been shown to be effective [34, 38]. The complementary diet also needs to be free of the food allergen. In formula-fed infants with cow’s milk allergy (CMA), specialized hypoallergenic formulas are the treatment of choice. The main types of these treatment formulas are EHF and amino acid-based formula (AAF) [40, 111, 112]. Hypoallergenic elimination diets need to be carefully supervised for nutritional adequacy [38]. Despite attempts to strictly eliminate offending food allergens from the diet, accidental reactions are relatively common. The risk of inadvertent allergic reactions and anaphylaxis significantly impacts the quality of life of patients and families [113, 114]. Precautionary allergen labelling is in many instances still confusing or incomplete [115, 116]. Reassuringly, several countries have introduced legislation towards more consistent allergen labelling [117].
Maternal Elimination Diets
Food allergens that are secreted into breast milk may elicit allergic symptoms in the infant [118]. While maternal elimination diets have no role in primary food allergy prevention, they have become a widely used intervention in breastfed infants with food allergies [38]. Poorly supervised or broad-based maternal elimination diets are not without nutritional risks for both mother and infant [119]. The nutritional adequacy of the maternal diet should be assessed and monitored by a pediatric dietitian [120]. Calcium supplementation is generally recommended if cow’s milk products are eliminated from the maternal diet.
Extensively Hydrolyzed Formula
Whey- or casein-based EHF are considered the firstline treatment of formula-fed infants with CMA [121]. These formulas contain small cow’s milk peptides that are produced via enzymatic breakdown and ultrafiltration of intact cow’s milk proteins. There are significant differences in the molecular weights and profiles of peptides in EHF. This may explain differences in the risk of allergic reactions to various EHF [122, 123]. A task force of the European Academy of Allergy and Clinical Immunology (EAACI) has therefore called for stricter standards for the definition of EHF marketed in Europe, including preclinical testing, quality assurance, and labelling requirements [124].
Some recently developed EHF contain highly purified lactose. Contrary to common perception, lactose is tolerated well by most infants with CMA [125]. The only exception are infants with cow’s milk protein-induced enteropathy and secondary lactase deficiency due to villous damage (Table 2). These infants may develop increased diarrhea after lactose ingestion. However, a lactose-containing EHF can generally be reintroduced once the diarrhea has settled and the small intestinal mucosal integrity has been restored. As young infants do not absorb all ingested lactose, it is considered a prebiotic compound with positive effects on the gut microbiome of infants [126]. Compared to lactose-free formula, lactose-containing formula is associated with increased counts of Bifidobacteria and increased concentrations of short-chain fatty acids. This may confer a protective effect on colonic mucosal integrity and have a beneficial effect on early immune development [53]. There is to date no data showing a direct effect on tolerance development or allergic risk.
EHF contains trace amount of allergenic peptides and therefore has a small residual allergenicity with the risk of allergic reactions [127]. Conversely, the antigenic content in EHF may have the potential to actively promote tolerance development [128]. This ability may be further enhanced by the addition of probiotic bacteria or other ingredients. Berni Canani et al. [129] conducted a randomized clinical trial of EHF supplemented with Lactobacillus rhamnosus GG (LGG) in infants with CMA. In that trial, infants receiving the probiotic-supplemented formula had a greater chance of achieving tolerance to cow’s milk protein at 6 and 12 months of age. This effect appeared to be, at least in part, modulated by an expansion of butyrate-producing gut microbiota [130]. At the 3-year fol- low-up of another cohort, there appeared to be a greater rate of resolution of IgE-mediated CMA as well as a lower incidence of other allergic manifestations in response to LGG-supplemented EHF [15]. These studies highlight the potential for probiotic supplementation of EHF to hasten tolerance development as well as the importance of butyrate as a likely key mediator in tolerance acquisition. However, further clinical trials are required to confirm the tolerogenic effects of LGG and assess the potential benefits of other probiotic strains with regard to early immune development.
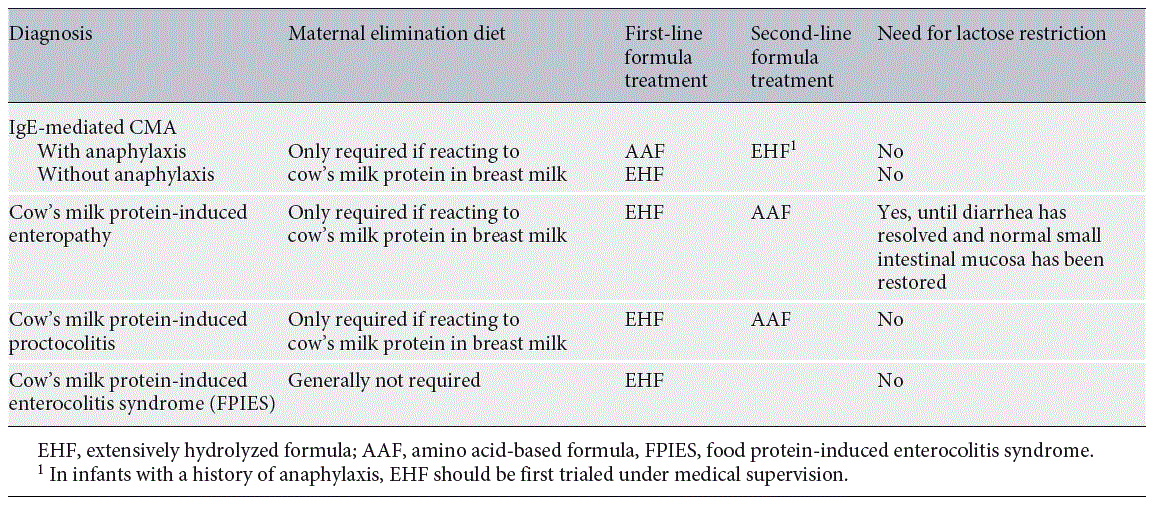
Table 2. Treatment of cow’s milk allergy (CMA) in infants
Amino Acid-Based Formula
AAF is a synthetic, nutritionally complete, cow’s milk antigen-free formula containing free amino acids, which is used in the treatment of infants with severe CMA. AAF is therefore not a first-line treatment but recommended for infants who have failed treatment with EHF, as well as infants with cow’s milk anaphylaxis [112], multiple food intolerance of infancy [36, 131], or eosinophilic esophagitis [132]. As tolerance development is thought to be an antigen-driven process [128], AAF is unlikely to promote tolerance development [128]. The addition of prebiotics or probiotics to AAF may have beneficial effects on gut microbiome, but clinical outcome data are not currently available [133].
Other Potential Formula Options
Should EHF or AAF not be available, other formula options may be considered. Soy formula is frequently used for economic reasons in countries with limited access to hypoallergenic formulas [134]. However, the role of soy formula in the treatment of infants with CMA remains controversial. Generally, soy formula is not recommended as a first-line treatment in infants with CMA under 6 months of age [112]. Hydrolyzed rice-based formula has become available in recent years as a hypoallergenic formula in infants with CMA [135, 136]. The hydrolysis is required due to the poor solubility and hydrophobic properties of rice protein. These formulas are tolerated well and may have a taste advantage over casein- or whey-based EHF. The exact role of hydrolyzed rice-based formulas needs to be clarified. Importantly, rice-based infant formulas must not be confused with rice “milk” beverages which are low-protein, low-energy cereal milks which are not suitable for infant feeding due to the risk of severe protein-energy malnutrition [137]. Mammalian milks, including goat’s or sheep’s milk formula, are often considered as alternatives to cow’s milk formula but are not suitable due to a high rate of protein homology and allergic cross-reactivity [138]. Cross-contamination with cow’s milk protein during production may also occur. Al- though often perceived as safe alternatives to cow’s milk formula, these mammalian milk formulas may cause significant allergic reactions, including anaphylaxis [139].
Food Allergen Immunotherapy
The concept of food allergen immunotherapy is not new. The concept was first described by Schofield in 1908 in a 13-year-old boy with egg allergy who was successfully desensitized by introducing egg in incremental doses [140]. Since then, 3 main clinical immunotherapy concepts to food allergens have emerged: oral, sublingual, and epicutaneous immunotherapy. The focus has so far mainly been on children with the most prevalent food allergies to peanut, egg, and cow’s milk.
Oral immunotherapy (OIT) involves the stepwise introduction of a food allergen via the oral route, starting with milligram doses [141] (Fig. 2). The administration of initial doses and stepwise updosing occurs under medical supervision, generally with fortnightly intervals, until a maintenance dose has been achieved after about 6 months [13]. For OIT to peanut, 79% of peanut-allergic patients were effectively desensitized to 443 mg of peanut protein [141]. The remaining patients were unable to tolerate the treatment due to significant allergic side effects, ranging from persistent gastrointestinal symptoms to anaphylaxis. Combination therapy of OIT with anti-IgE (omalizumab) or other biologicals is being explored as an avenue to reduce the rate and severity of adverse events during updosing [142]. Importantly, patients generally do not achieve true, lasting immunological tolerance but a state of non-responsiveness, called “desensitization.” The duration of “sustained unresponsiveness” to an allergen after cessation of OIT maintenance treatment varies, but the allergic phenotype generally recurs gradually over weeks to months [13].
Epicutaneous immunotherapy (EPIT) is based on the delivery of food allergens via intact skin [143]. The allergen is bound to a thin plastic membrane that is placed onto the skin under an occlusive patch, similar to the approach for atopy patch testing [144, 145]. The allergen is taken up by Langerhans cells in the epidermis. These are immune-competent antigen-presenting cells that have the ability to initiate a regulatory T-cell response and communicate with regional lymph nodes. Daily application of EPIT patches containing 250 μg of peanut for 12 months in patients with peanut allergy has been shown to significantly raise the threshold dose for allergic reactions on food challenge [146, 147]. The desensitization effect is not as marked as that of OIT, but the rate of adverse effects is minimal and mainly limited to local skin irritation where the EPIT patch has been applied [143]. Guidelines for the clinical use of OIT versus EPIT in clinical practice still have to be defined.
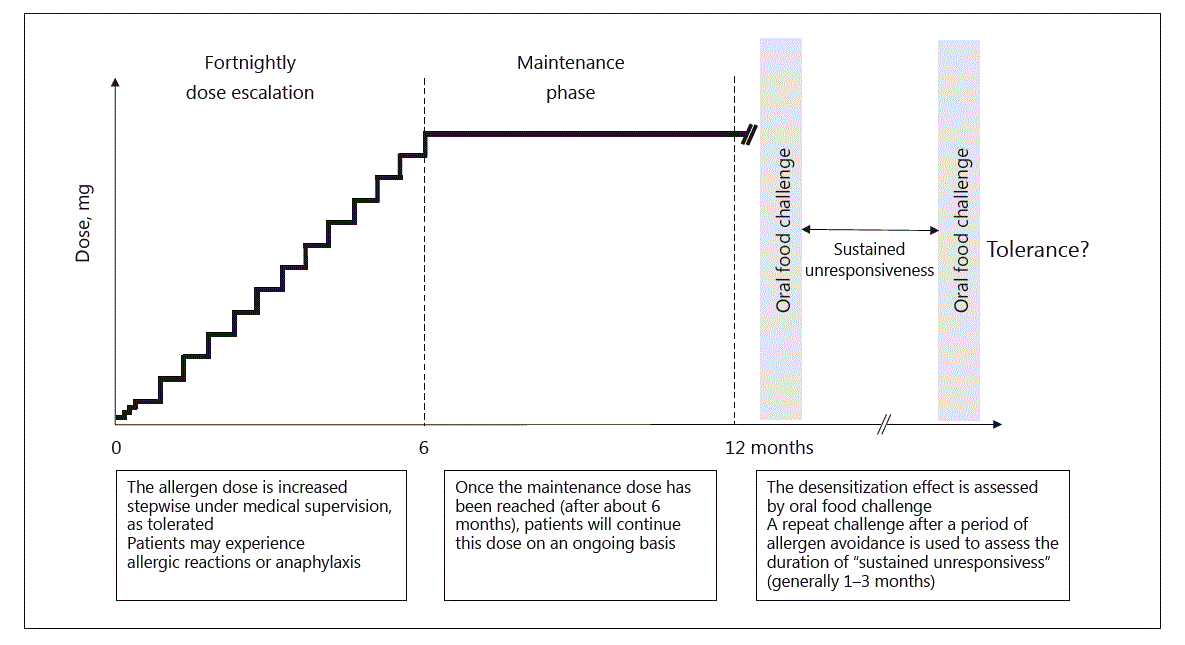
Fig. 2. Dosing schedule of oral peanut immunotherapy.
Conclusion
Based on recent research, food allergy prevention and treatment have undergone significant improvements. Further research is needed to inform the most effective food allergy prevention strategies at the population level (Table 1). Effective prevention has the potential to reverse the rising prevalence trends for food allergies. In addition, the wider application of food allergen immunotherapy may provide better health outcomes and improved quality of life for families affected by food allergies.