Fatty Acids and Fat-Soluble Vitamins in Breast Milk: Physiological Significance and Factors Affecting Their Concentrations
Abstract
Fatty acids (FAs) and fat-soluble vitamins are vital components of the human milk lipid fraction. About two-thirds of the human milk FA fraction consist of oleic, linoleic, and palmitic FAs, but the precise composition depends on maternal geography, diet, and genetics. Mothers with high fish consumption have more docosahexaenoic acid (DHA) and other ω-3 FAs in their milk, while mothers with high dairy consumption have more branched-chain FAs in their milk. Vitamins A and E are the most common fat-soluble vitamins, but milk concentrations vary, depending on maternal diet and body stores. Vitamin D is typically low or undetectable in mother’s milk and typically fails to meet the infant needs. However, trial data indicate that high maternal supplementation (6,400 IU/ day) safely provides nutritionally adequate amounts of vitamin D in her milk. FA and fat- soluble vitamin levels in mother’s milk can significantly influence infant health; for ex- ample, in preterm infants, low endogenous stores of DHA paired with low levels in maternal milk may influence the risk of chronic lung disease and other inflammatory conditions. Greater attention is warranted to the variation in FA and fat-soluble vitamin content of human milk in relation to infant health.
Introduction
Fatty acids (FAs) and fat-soluble vitamins are key components of the lipid fraction of human milk. Lipid is the second-most abundant solid constituent of human milk after lactose, but it is also the most highly variable macronutrient of milk [1]. Milk expressed late in a feed or pumping episode contains as much as 2–8 times more fat than at the start of the feed [1, 2]. In addition, lipid content is also reportedly lower in night and morning than afternoon or evening feeds [1, 3]. Given this high variability, accurate study of lipid-associated components requires accounting for sample lipid content.
Human milk FA composition has a core similarity (Fig. 1) but differs across populations in the abundance of many FAs, influenced by maternal diet and genetics (Table 1). High interindividual and population variability in docosahexaenoic acid (DHA) and other n-3 FAs of human milk is often observed [4–7]. Other FAs that differ between populations include the trans-FAs, and the n- 6/n-3 ratio of polyunsaturated FAs (PUFAs) [6]. We have also reported that the branched-chain FAs (BCFAs) of human milk differ between populations [7]. Evidence indicates that the FA dietary profile of infants influences the risk of inflammatory conditions and neurodevelopment, and is relevant to later-life cardiovascular health [8].
The fat-soluble vitamins are also important contributors to the lipid fraction of human milk. Human milk typically has adequate levels of fat-soluble vita- mins A and E to meet infant needs (Table 2), though there is variation between populations. However, human milk is typically low in vitamins D and K. Vitamin D supplementation of 400 IU/day for all infants is a current global consensus recommendation by 11 international scientific organizations for the prevention and management of nutritional rickets [9]. Fat-soluble vitamins perform important health functions and can be stored in the liver and fat tissue until required. Because they are fat soluble, these vitamins are absorbed from the diet through the small intestine along with dietary fat and are readily stored for use. Below, we briefly review the FAs, followed by the fat-soluble vitamins of human milk.
Human Milk Fatty Acids
Description
FAs are carboxylic acids with long aliphatic chains. In human milk, the FAs are found in saturated, monounsaturated, polyunsaturated, and branched forms. The preponderance of human milk FAs are long-chain FAs, which include tails of 13–21 carbons, but, human milk also includes medium-length FAs, including 8–12 carbon tails, and very-long-chain FAs, with tails of 22 or more carbons. Compared to cow’s milk, human milk contains a higher proportion of PUFAs and long-chain PUFAs (LCPUFAs) [1]. Most human milk FAs are unbranched, but human milk also contains forms of BCFAs ranging from 14 to 18 carbon chains [7]. About two-thirds of human milk fat is composed of 3 major FAs: oleic (c18:1 n-9, a monounsaturated FA); palmitic (c16:0, a saturated FA); and linoleic (c18:2 n-6, a PUFA). While these 3 FAs are consistently dominant, the exact FA quantities and profile of human milk otherwise varies significantly be- tween mothers and populations (Fig. 1) [6, 7].
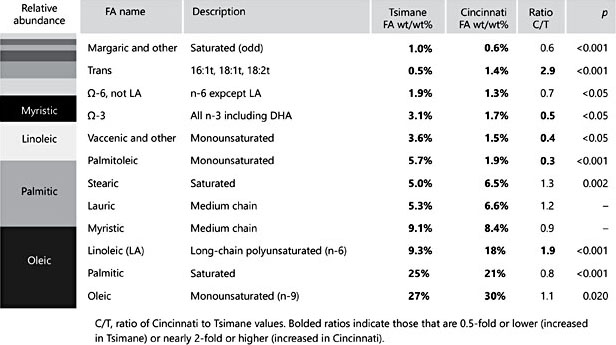
Fig. 1. Abundance of fatty acids (FAs) in human milk taken from late lactation, comparing a Bolivian forager population (the Tsimane) to an urban US midwestern population (Cin- cinnati, OH, USA). Bar chart on the left represents the relative abundance of specific FAs in the order listed in the table to the right. In both populations, about two-thirds of the FAs consist of oleic, palmitic, and linoleic acids. In other ways, the relative abundance pro- file differs by population (adapted from Martin et al. [6])
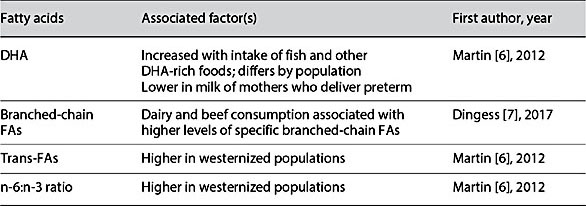
Table 1. Dietary factors associated with varying concentrations of fatty acids (FAs) in human milk
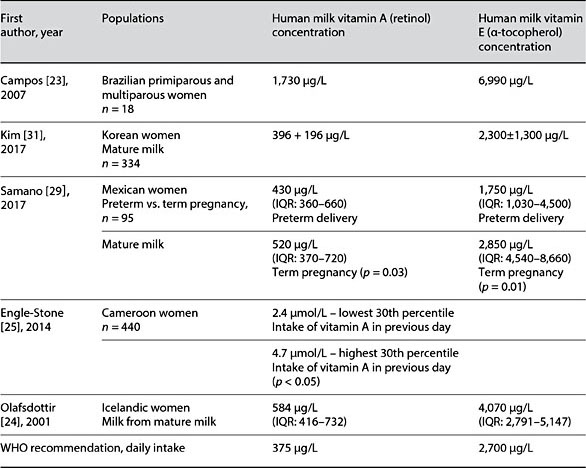
Table 2. Reported human milk vitamin A and E values in global studies
Factors Affecting Varied Concentrations
Many FAs of human milk vary between populations, but some vary more than others. DHA is one of the most-well-studied FAs of human milk. DHA (C22:6) is a critical n-3 PUFA, and its contribution to human milk content is significantly lower in populations with low DHA dietary intake. Martin et al. [6] re- ported twofold greater levels of DHA and other n-3 FAs in the milk of the Tsimane, a Bolivian forager population, compared to mothers residing in Cincinnati, OH, an urban, midwestern US city. Consistent with known differences in diet, the milk of Cincinnati mothers had a significantly higher ratio of n-6/n-3 FAs, and twofold increased linoleic acid and total trans-FAs compared to Tsimane mothers. Differences in FA composition have been observed within the United States. A comparison of donor human milk from 6 milk banks across the US found a trend towards linoleic and other FA profile differences in individual milk samples donated from different regions of the United States [5]. In a study of human milk FA composition in the US over nearly 60 years, Ailhaud et al.[10] reported a threefold rise in linoleic acid between about 1945 and 2005. Thus, some of the differences now observed between populations may be due to relatively recent changes in dietary fat sources.
We compared FAs of milk from mothers in Shanghai, China; Mexico City, Mexico and Cincinnati, OH, USA and identified another intriguing population level difference: The BCFA content was highest in women residing in Cincinnati, followed by women in Mexico, and lowest among women in Shanghai. Higher dietary intake of dairy foods was significantly associated with higher levels of the BCFAs iso C14:0, anteiso C15:0, and iso C16:0. Higher beef intake was associated with significantly higher levels of the BCFA iso C16:0 in human milk [7].
In addition to diet, the LCPUFA composition of human milk can also be influenced by polymorphism in maternal FA desaturase (FADS) genes, which are involved in FA elongation. In humans, FADS1 and FADS2 genes influence the ability to synthesize LCPUFAs; thereby, they modify the concentration of LCPUFA in human milk, and, specifically, the impact of fish intake on the DHA composition of human milk [11, 12]. However, FADS genes appear to be important only when the endogenous synthesis of LCPUFAs provides a compensatory advantage, that is, only when dietary intake of DHA or other LCPUFAs are limited. When the level of DHA is low in human milk, it can be dramatically increased by dietary supplementation with preformed DHA [4], regardless of the genetics of the mother.
It is noteworthy that proteomic analysis of samples from 3 global populations [13]found that FA synthesis proteins consistently increase over the course of lactation. This finding suggests that early in lactation, the FAs found in human milk may be derived more from direct blood influx (dietary sources), while late in lactation, human milk FAs may be derived more from de novo mammary synthesis (which may indicate a greater potential genetic influence).
Physiological Effects
Human milk FA composition may have a powerful influence on infant health. FAs regulate intracellular signaling and affect inflammatory response, cardiovascular development, and central nervous system development and function [14]. Thus, it is intriguing that infants worldwide can be exposed to significantly different FA profiles in their mother’s milk. The current Western diet may be reasonably rep- resented by Cincinnati mothers, who have high BCFA, linoleic acid, and trans-FA contents, and low ω-3 and monounsaturated FA contents in their milk, than the milk of women in Tsimane, who represent the traditional dietary pattern. The physiological or metabolic impact these differences remains to be determined.
There is strong and growing evidence, however, that the FA content of mother’s own milk and donor milk are insufficient to meet recommendations for the health and nutrition in preterm infants. Several randomized, controlled trials have reported that supplementation of pregnant mothers with DHA contributes to longer gestation and greater infant birth weight [14]. Consistent with that finding, DHA levels are low in the milk of preterm infants [4, 15]. Further, it was found that the level of DHA measured in donor milk was also too low to support the nutritional needs of preterm infants. Preterm infants are at further risk of DHA deficiency because the accretion of DHA occurs in utero predominantly during the last trimester of pregnancy, a period that preterm infants have missed. The lack of endogenous DHA stores and low DHA levels in maternal or donor milk appear to place preterm infants at high risk of adverse outcomes during their hospitalization. In a cohort of preterm infants <30 weeks gestation, low DHA levels and increased linoleic acid/DHA ratios were associated with chronic lung disease and late onset of sepsis [15]. These and other findings provide a strong argument for attending to maternal and infant FA nutrition, including the FA composition of human milk.
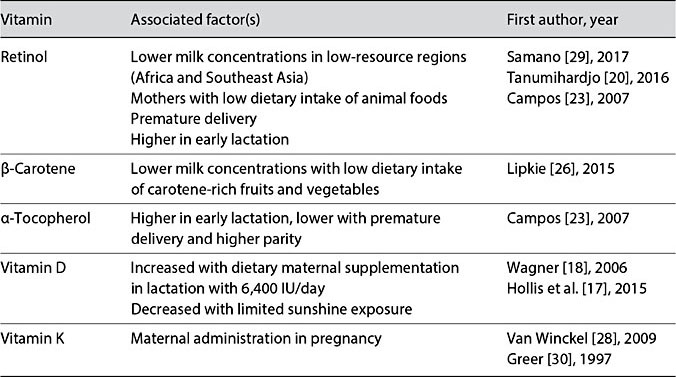
Table 3. Factors associated with varying concentrations of fat-soluble vitamins in human milk
Fat-Soluble Vitamins of Human Milk
Description
The fat-soluble vitamins – vitamins A, D, E, and K – are critical to infant health. Fat-soluble vitamins are absorbed from the diet through the small intestine along with dietary fat. They are readily stored for use and tend to persist in the body. Levels of fat-soluble vitamins in human milk are thus typically stable. The quantities of vitamins A and E in human milk appear typically adequate to meet infant needs, though limited in some vitamin-A-deficient mothers (Table 2). However, vitamin D is typically absent in human milk [16], though recent data indicate that vitamin D supplementation of lactating women with 6,400 IU/day can safely produce adequate levels of milk vitamin D to satisfy the requirements of nursing infants [17, 18]. Like Vitamin D, vitamin K is also low in human milk and typically provided directly to newborns.
Vitamin A
Vitamin A refers to a set of related compounds that include preformed vitamin A and provitamin A carotenoids. Once consumed, these forms are converted and stored as retinol, which is used as the measure of vitamin A equivalence. Preformed vitamin A is predominantly obtained from the liver, fish oil, milk, and eggs. The provitamin A carotenoids are dietary vitamin A precursors obtained from plant foods. The most important provitamin A carotenoid is β-carotene. α-Carotene and β-cryptoxanthin also contribute some provitamin A activity.
Vitamin A plays a key role in vision, bone growth, reproduction, immunity, cell development, and skin health. Retinol and its metabolites regulate many functions in the body, including maintenance of epithelial cell integrity [19]; expression of genes that encode structural proteins, enzymes, extracellular matrix proteins, and retinol-binding proteins and receptors; and maintenance of immune function [20]. In the eye, these molecules are responsible for the differentiation of the cornea and conjunctiva, for the activity of retinal photoreceptor cells, and for changing light to neural signals for vision. Retinol and its metabolites are especially critical in early development.
Vitamin A deficiency remains a major health problem of low-resource countries and results in impaired resistance to infection, xerophthalmia, blindness, and increased risk of mortality (Table 3). As many as 190 million preschool-aged children and 19 million pregnant women suffer from vitamin A deficiency according to WHO estimates [21, 22]. Based on observations of breastfed infants in communities in which good nutrition is the norm, WHO set the recommended dietary intake for infants <6 months as 375 μg retinol equivalents (RE) per day. For an exclusively breastfed infant consuming between 650 and 750 mL per day, meeting the target intake could require a milk concentration as high as 500–600 μg/L RE/day. In healthy women with adequate vitamin A nutrition, these levels may be exceeded (Table 2) [23, 24], while in some populations, reported concentrations appear to be just meeting or modestly below the WHO- recommended intake (Table 2). In some low-resource regions, as reported in Cameroon [25], concentrations of retinol may be low among women with limited dietary sources of vitamin A. Despite finding vitamin A levels in the milk of a vitamin-A-deficient mother to be less than ideal in such populations, they are considered adequate to help reduce the risk of xeropthalmia in the infant [20]. Prenatal vitamin supplementation is effective in increasing maternal serum and breast milk concentrations [3]. In populations at high risk of vitamin A deficiency, maternal supplementation programs have focused on pregnant mothers and infants after 6 months of age. In preterm infants in high-resource countries, vitamin A supplementation of the infant during hospitalization is a priority, as preterm infants are typically born with low vitamin A stores.
The vitamin A content of human milk varies also in relation to the dietary sources of vitamin A. Analysis of provitamin A carotenoids in milk samples from China, the US, and Mexico found that the most abundant provitamin A carotenoids was β-carotene, followed by β-cryptoxanthin and α-carotene [26]. Chinese mothers had significantly higher levels of carotenoids in their milk than US and Mexican mothers, likely due to maternal dietary differences (Table 3). However, while these carotenoids contribute to the total retinol activity of human milk, they are far less abundant and efficient than retinol to support the retinol activity of human milk.
Vitamin D
Worldwide, studies of vitamin D in human milk have found concentrations to be below detectable levels. Inadequate vitamin D nutrition results in poor bone health and increased risk of infection. Given the absence of vitamin D in breast milk, breastfed infants are at increased risk of vitamin D deficiency, with occurrence of its most severe form – rickets – in many populations. Thus, the global public health recommendation has been to provide breastfed infants with vita- min D supplements after birth to prevent vitamin D deficiency and provide essential support for calcium absorption and bone growth [9]. Recent studies provide an alternative strategy. In a randomized, controlled trial, mothers given 6,400 IU of vitamin D during lactation achieved clinically adequate amounts of vitamin D in their milk to satisfy infant needs during early infancy (Table 3) [17]. Nevertheless, the recommended public health strategy at this time remains direct supplementation of the breastfed infant with vitamin D.
Vitamin E
It is comprised of 8 isoforms, including 4 tocopherol isoforms: α-, γ-, β-, and δ-tocopherol. Of these, α- tocopherol is the dominant form of vitamin E in human milk, followed by γ- tocopherol. Vitamin E is a potent antioxidant that protects against free radicals, molecules that cause cellular damage. Vitamin E is also reported to benefit immune health and serves to reduce the risk of blood clot-
ting. α- and γ-tocopherol differ by 1 methyl group and have a similar capacity to scavenge reactive oxygen species, but γ-tocopherol may serve as a more po- tent antioxidant due to its capability to react with reactive nitrogen species. However, α-tocopherol is found at higher concentrations in milk and tissues than γ-tocopherol, likely due to the preferential transfer of α-tocopherol to lipid particles by liver α-tocopherol transfer protein [27].
WHO recommends an infant intake of vitamin E of 2,700 μg/day. Typically, concentrations of vitamin E in human milk from different populations meet this recommendation such that mothers are able to provide this quantity to their ex- clusively breastfed infants per day (Table 2). However, vitamin E levels have been reported to be considerably higher than the WHO recommendation in some populations [23, 24]. In other populations, e.g., in mothers who have de- livered a preterm infant, vitamin E levels may be somewhat lower than recom- mended (Table 3). The possible impact of lower vitamin E levels on infant health outcomes is understudied.
Vitamin K
Vitamin K is responsible for the carboxylation of proteins that bind calcium, which is required for normal coagulation. Thus, vitamin K deficiency can be dangerous and result in delayed coagulation and vitamin K deficiency bleeding. For exclusively breastfed infants, the two sources of vitamin K are mother’s milk and their own endogenous gut bacteria. Human milk is a poor source of vitamin K, containing only 1–4 μg/L. The recommended dietary intake of vitamin K in infancy is 1 μg/kg body weight/day, which translates to a daily requirement of 5–10 μg/day, a requirement rarely met by human milk consumption. A single placebo-controlled trial showed that supplementing lactating mothers with high-dose vitamin K (5 mg/day) increases the level in their breast milk and is associated with an improved protein carboxylation profile (Table 3) [28–30]. Nevertheless, to assure prevention of early vitamin K deficiency bleeding of the newborn, administration of vitamin K to the newborn is the standard of care.
Conclusion
Exclusively breastfed infants rely on mother’s milk to meet their needs. The study of diverse populations has elucidated the compositional description of human milk components and basic understanding of factors that influence human milk composition. In relation to the FAs, 3 FAs consistently form the major part of the FA fraction (oleic, palmitic, and linoleic acids). Nevertheless, considerable variation is seen between populations in the quantity of specific FAs. This variation is largely due to maternal dietary differences, though genetic polymorphisms can contribute when dietary intakes of specific LCPUFAs are limited. In relation to the fat-soluble vitamins, vitamins A and E are typically present in robust quantities in human milk, though some high-risk mothers (e.g., those who deliver preterm or live in resource-poor countries) have lower than recommended levels. However, vitamins D and K are typically low in human milk, and infant supplementation is the recommended strategy for assuring adequate infant nutrient status. Our review of the literature suggests that the most critical scientific questions are: Do variable levels of FAs in human milk impact the health or development of infants? This question is also pertinent for some of the fat-soluble vitamins of human milk. Greater attention is also warranted to maternal supplementation as an approach to modifying FA levels and fat-soluble vitamins in human milk, particularly in relation to DHA and vitamin D.
References
-
1 Ballard O, Morrow AL: Human milk composition: nutrients and bioactive factors. Pediatr Clin North Am 2013;60:49–74.
-
2 Hassiotou F, Hepworth AR, Williams TM, et al: Breastmilk cell and fat contents respond similarly to removal of breastmilk by the infant. PLoS One 2013;8:e78232.
-
3 Sanzio Gurgel CS, Alves de Araujo Pereira L, de Assis Costa A, et al: Effect of routine prenatal supplementation on vitamin concentrations in maternal serum and breast milk. Nutrition 2017;33:261–265.
-
4 Valentine CJ, Morrow G, Pennell M, et al: Randomized controlled trial of docosahexaenoic acid supplementation in midwestern U.S. Human milk donors. Breastfeed Med 2013;8:86–91.
-
5 Valentine CJ, Morrow G, Reisinger A, et al: Lactational stage of pasteurized human donor milk contributes to nutrient limitations for infants. Nutrients 2017;9:E302.
-
6 Martin MA, Lassek WD, Gaulin SJ, et al: Fatty acid composition in the mature milk of Bolivian forager-horticulturalists: controlled comparisons with a US sample. Matern Child Nutr 2012;8:404–418.
-
7 Dingess KA, Valentine CJ, Ollberding NJ, et al: Branched-chain fatty acid composition of human milk and the impact of maternal diet: the Global Exploration of Human Milk (GEHM) Study. Am J Clin Nutr 2017;105:177–184.
-
8 Mozaffarian D, Wu JH: (n-3) fatty acids and cardiovascular health: are effects of EPA and DHA shared or complementary? J Nutr 2012; 142:614S–625S.
-
9 Munns CF, Shaw N, Kiely M, et al: Global consensus recommendations on prevention and management of nutritional rickets. J Clin Endocrinol Metab 2016;101:394–415.
-
10 Ailhaud G, Massiera F, Weill P, et al: Tempo- ral changes in dietary fats: role of n-6 polyun- saturated fatty acids in excessive adipose tissue development and relationship to obesity. Prog Lipid Res 2006;45:203–236.
-
11 Molto-Puigmarti C, Plat J, Mensink RP, et al: FADS1 FADS2 gene variants modify the association between fish intake and the docosa- hexaenoic acid proportions in human milk. Am J Clin Nutr 2010;91:1368–1376.
-
12 Xie L, Innis SM: Genetic variants of the FADS1 FADS2 gene cluster are associated with altered (n-6) and (n-3) essential fatty acids in plasma and erythrocyte phospholipids in women during pregnancy and in breast milk during lactation. J Nutr 2008;138:2222– 2228.
-
13 Zhang Q, Cundiff J, Maria S, et al: Quantitative analysis of the human milk whey proteome reveals developing milk and mammary-gland functions across the first year of lactation. Proteomes 2013;1:128–158.
-
14 Carlson SE, Colombo J, Gajewski BJ, et al: DHA supplementation and pregnancy out- comes. Am J Clin Nutr 2013;97:808–815.
-
15 Martin CR, Dasilva DA, Cluette-Brown JE, et al: Decreased postnatal docosahexaenoic and arachidonic acid blood levels in premature infants are associated with neonatal morbidities. J Pediatr 2011;159:743–749.e1–2.
-
16 Dawodu A, Tsang RC: Maternal vitamin D status: effect on milk vitamin D content and vitamin D status of breastfeeding infants. Adv Nutr 2012;3:353–361.
-
17 Hollis BW, Wagner CL, Howard CR, et al: Maternal versus infant vitamin D supplementation during lactation: a randomized controlled trial. Pediatrics 2015;136:625–634.
-
18 Wagner CL, Hulsey TC, Fanning D, et al: High-dose vitamin D3 supplementation in a cohort of breastfeeding mothers and their infants: a 6-month follow-up pilot study. BreastfeedMed 2006;1:59–70.
-
19 Gudas JM, Oka M, Diella F, et al: Expression of wild-type p53 during the cell cycle in normal human mammary epithelial cells. Cell Growth Differ 1994;5:295–304.
-
20 Tanumihardjo SA, Russell RM, Stephensen CB, et al: Biomarkers of Nutrition for Development (BOND) – vitamin A review. J Nutr 2016;146:1816S–1848S.
-
21 Novotny JA, Harrison DJ, Pawlosky R, et al: β-Carotene conversion to vitamin A decreases as the dietary dose increases in humans. J Nutr 2010;140:915–918.
-
22 WHO Global Prevalence of Vitamin A Deficiency in Populations at Risk 1995–2005: WHO Global Database on Vitamin A Deficiency. Geneva, WHO, 2009.
-
23 Campos JM, Paixao JA, Ferraz C: Fat-soluble vitamins in human lactation. Int J Vitam Nutr Res 2007;77:303–310.
-
24 Olafsdottir AS, Wagner KH, Thorsdottir I, et al: Fat-soluble vitamins in the maternal diet, influence of cod liver oil supplementation and impact of the maternal diet on human milk composition. Ann Nutr Metab 2001;45: 265–272.
-
25 Engle-Stone R, Haskell MJ, Nankap M, et al: Breast milk retinol and plasma retinol-binding protein concentrations provide similar estimates of vitamin A deficiency prevalence and identify similar risk groups among women in Cameroon but breast milk retinol underestimates the prevalence of deficiency among young children. J Nutr 2014;144:209– 217.
-
26 Lipkie TE, Morrow AL, Jouni ZE, et al: Longitudinal survey of carotenoids in human milk from urban cohorts in China, Mexico, and the USA. PLoS One 2015;10:e0127729.
-
27 Marchese ME, Kumar R, Colangelo LA, et al: The vitamin E isoforms α-tocopherol and γ-tocopherol have opposite associations with spirometric parameters: the CARDIA study. Respir Res 2014;15:31.
-
28 Van Winckel M, De Bruyne R, Van De Velde S, et al: Vitamin K, an update for the paediatrician. Eur J Pediatr 2009;168:127–134.
-
29 Samano R, Martinez-Rojano H, Hernandez RM, et al: Retinol and α-tocopherol in the breast milk of women after a high-risk pregnancy. Nutrients 2017;9:E14.
-
30 Greer FR, Marshall SP, Foley AL, et al: Im- proving the vitamin K status of breastfeeding infants with maternal vitamin K supplements. Pediatrics 1997;99:88–92.
-
31 Kim H, Jung B-M, Bum-Lee N, Kim Y-J, Jung JA, Chang N: Retinol, α-tocopherol, and selected minerals in breast milk of lactating women with full-term infants in South Korea. Nutr Res Pract 2017;11:64–69.