Early-Life Nutrition and Microbiome Development
Abstract
Recent demonstrations link clinical conditions, phenotypes alternating from inflammatory bowel disease, obesity, and allergic diseases to neurodevelopmental disorders, to aberrant gut microbiota composition. This has led to a growing interest in host-microbe crosstalk, characterizing the healthy microbiome and modifying its deviations at an early age. The rationale arises from the recognition of the intimate interrelationship between diet, immune system, and microbiome and the origins of human diseases. Before satisfactory preventive measures can be put in practice, important questions remain to be solved. First, we need more profound understanding of the complex mechanisms underlying these heterogeneous manifestations of immune-mediated and microbiome-associated chronic conditions. Second, long-term follow-up studies are required to determine whether the changes in the microbiome underlie the pathogenesis of noncommunicable dis- eases or are merely end results thereof, confronting the question of causality. This uncertainty notwithstanding, the complex and bidirectional interrelationship of the diet and the gut microbiota is becoming evident. Early exposures by the enteral route induce dynamic adaptive modifications in the microbiota composition and activity, which may carry long-term clinical impacts. Microbiota changes, again, control energy acquisition and storage and may contribute to gut immunological milieu; high-energy Western diets alter the microenvironment of the gut leading to propagation of the inflammatory tone and perturbation of gut barrier function and thereby to systemic low-grade inflammation. On this basis, rigorous clinical intervention studies, providing the ultimate answers to these questions, need accurate characterization of the immediate environment of the child, in particular the early nutrition. The model of early nutrition for future studies is the healthy breastfed infant that remains healthy in the long term. Scientific interest is currently extending from the duration of breastfeeding to the composition of breast milk, which shows marked variation according to the mother’s immunological and metabolic health, antibiotic use, and mode of delivery. Human milk, rich in bioactive compounds, including health-promoting microbes and their optimal growth factors, human milk oligosaccha- rides, continues to afford tools to study diet-microbiota interactions for research aiming at reducing the risk of noncommunicable diseases.
The Gut Barrier and the Healthy Microbiota
The mucosal surface of the gastrointestinal tract forms an important organ of host defense. In addition to its main physiological function, digestion and absorption of nutrients to meet the metabolic requirements and the demands of normal growth and development, the intestinal mucosa provides a protective interface between the internal environment and the constant challenge from antigens such as food and microorganisms from the outside environment. The maturation of balanced immunophysiological regulation here, however, depends on these external stimuli, particularly on the initial establishment of the gut microbiota. In point of fact, microbe contact in the perinatal period represents the most massive antigen exposure educating the physiological adaptation processes to the awaited postnatal environment.
One theory of the emergence of noncommunicable diseases involves disintegration in the maturation of the host key regulatory systems vis-à-vis the gut colonization process. Indeed, the microbiome is sensitive to environmental ex- posures displaying rapid adaptive competence, unlike the host genome [1]. The adaptations to an altered environment involve dynamic modifications in the microbiota composition and activity.
On this basis, the definition the healthy versus aberrant gut microbiota compo- sition is unfeasible without considering the immediate environment of the child. Indeed, fundamental determinants of the infant gut colonization include maternal health and nutrition, the mode of delivery, early feeding, and antibiotic use.
Gut Microbiota: A Target for Preventive and Therapeutic Measures?
The industrialized societies worldwide are facing epidemics of diet-related chronic diseases, noncommunicable diseases, such as allergic, autoimmune, and inflammatory diseases. These conditions have been inextricably associated with an aberrant compositional development of gut microbiota, dysbiosis. Furthermore, early-life exposures that are known to perturb gut colonization, including cesarean section delivery and antibiotic use, have been consistently linked to increased risk of noncommunicable disease [2, 3]. Especially during critical stag- es of development, dysbiosis induces lasting alterations in the immune and metabolic phenotype as well as neural pathways [4, 5], example manifestations including obesity, type 1 diabetes, asthma, allergies, and even neurodevelopmental disorders [6].
However, our knowledge of the cascade of events underlying the pathophysiology of these conditions with different target organs, onset age, and prognosis is by no means satisfactory. We need more profound understanding of the complex mechanisms underlying these heterogeneous manifestations of immune-mediated and microbiome-associated chronic conditions. Importantly, the question of causality needs to be given top priority in future research activities [7].
Thus, the microbiota forms a moving target for any preventive and therapeutic measures, as we do not know whether the changes in the microbiome under- lie the pathogenesis of noncommunicable diseases or are merely a result thereof. Indisputably, the proof of causality requires clinical intervention studies in humans in different populations with rigorous and detailed documentation of the environment the infant is exposed to, the major determinant being early nutrition.
Infant Gut Microbiota: Origin and Determinants of the Composition
While the colonization process in the infant gut has been intensively studied, the early events guiding the microbiome development have only recently become uncovered. A stepwise process can characterize the establishment of the gut mi- crobiota (Fig. 1). The initial inoculum before, at, and following birth involves mainly facultative anaerobic bacteria. Indeed, recent advances indicate that microbial colonization of the gut may start already during the fetal period. The first colonizing microbes present in amniotic fluid can also be recovered in meconium and belong to the Escherichia genus and lactic acid bacteria, including members of the genera Leuconostoc, Enterococcus, and Lactococcus [8].
Thereafter, the exposure to specific species in neonates is facilitated by the mode of delivery: vaginally delivered newborns harbor microbes from the vagina including Prevotella and Lactobacillus and also the genera Bacteroides, Bi- fidobacterium, Parabacteroides, and Escherichia. The maternal gut also appears to be an important source of early colonizing bacteria: 72% of gut bacteria in vaginally delivered newborns are of maternal intestinal origin versus 41% in subjects born by cesarean section [9]. Indeed, newborns delivered by cesarean section are frequently colonized by bacteria associated with the maternal skin and mouth or the environment [9, 10]. Cohort studies from Europe and North America document reduced fecal abundance of Bacteroides or reduced diversity of Bacteroidetes phylum in infant gut following cesarean section delivery [11, 12]. In these, the fecal microbiome contains among others Enterobacter, Staphylococcus, including S. aureus, Streptococcus, and Veillonella, while Bifidobacterium are less abundant in cesarean-born than vaginally born infants [11, 12]. Cesarean-born infants also harbor more Clostridium difficile, and this may be mediating the dysbiosis frequently detected in these [12].
This initial colonization process directs the later microbiota succession and health of the infant [13]. Later in infancy, Bacteroides, Bifidobacterium, Parabacteroides, and Escherichia/Shigella species are abundant [9]. Maternal intrapartum antibiotic therapy or prophylaxis has also been reported to significantly perturb early gut colonization patterns and result in reduced diversity and low abundance of Actinobacteria among early gut microbiota [14, 15]. The detrimental impact of intrapartum antibiotic exposure appears to be most pronounced in infants born by cesarean section delivery [14, 15]. Indeed, long-term follow-up study shows that the impact of the mode of birth on gut microbiota composition may be intensified if broad-spectrum antibiotics were used during infancy. Neonatal antibiotic exposure has been reported to result in increased abundance of Proteobacteria and Firmicutes and reduced abundance of Bacteroidetes and Actinobacteria, particularly bifidobacteria, during the first weeks of life [15–18].
After birth, the sources of environmental exposure directly shaping the risk of disease are mainly associated with breastfeeding and, more specifically, the breast milk composition. Breast milk favors the predominance of bifidobacteria in the infant gut. Undeniably, the most important step of the colonization process comprises a rapid succession by anaerobic genera such as Bifidobacterium,Eubacterium, Clostridium, and increases in Bacteroides species. Different species of Bifidobacterium can reach up to 90% of the total fecal microbiota in breastfed infants, and frequently the composition comprises B. breve, B. infantis, and B. longum species, whereas the most common Lactobacillus in breast- and formula-fed infant feces are usually related to the Lactobacillus acidophilus group. Breastfeeding also exposes the infant to the mother’s skin bacteria. Recent study has shown the mean contribution of breast milk and areolar skin to the infant microbiome is 27.7% (SD 15) and 10.3% (SD 6.0), respectively [19]. Perez et al.[20]have shown that some bacterial signatures in breast milk are common to the infant’s feces and mother’s blood samples, which would imply a link. Hence, the breast milk microbiota contains a distinct bacterial community from skin, gut, vagina, or mouth.
A major step in the gut colonization coincides with weaning and subsequently towards microbial consortia characteristic of the adult microbiota by the age of 3 years [21, 22]. After weaning, the differences between breast- and formula- fed infants disappear due to the increase in the numbers of Bacteroides, Clostridium, and other anaerobic cocci in the former group, with general increases in numbers of E. coli and enterococci after weaning in both groups. During this step, rapid changes take place particularly in energy-harvesting Bacteroides species, this presumably reflecting the diet and the health of the host [21].
Following the first year of life, the rapid shift in microbiota composition and activity continues, and the community becomes more diverse, with Bacteroides, Veillonella, and Fusobacterium on the increase. At the same time, the number of unculturable microbes also increases, posing a challenge in characterizing the composition and the activity of the total microbiota. Nevertheless, it has been reported that children may still harbor higher numbers of bifidobacteria and enterobacteria than adults [21].
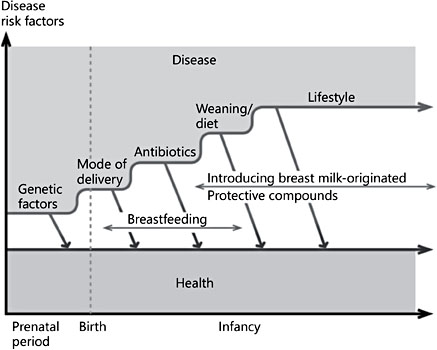
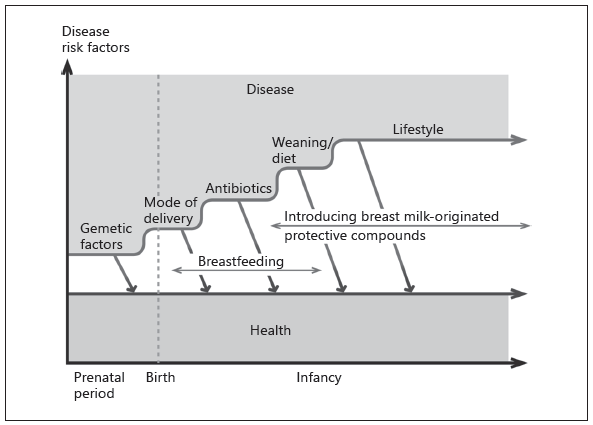
Fig. 1. The progression of gut colonization and the child’s risk of developing noncommunicable diseases. Key risk factors during the perinatal period and infancy include unfavorable nutritional environment during pregnancy, being born preterm or by cesarean section, or being devoid of important immunomodulatory compounds of breast milk. Resil- ience to unfavorable changes during this critical period of maturation may be achieved by endorsing breastfeeding and introduction of active protective compounds.
Optimal Nutrition for the Healthy Microbiome
The foundation of nutrition lies in a healthy, balanced diet to meet the needs for growth and development in children. Research interest in pediatric nutrition is currently directed beyond the nutritional impact of food towards the potential to reduce the risk of diseases, preferably benefiting from the concept of personalized nutrition. This is also the focus for microbiome research of specific active compounds with a documented capacity to strengthen the endogenous host defenses and to avert dysbiosis at an early age. However, the purpose is not to alter the gut microecology per se, but to adjust proinflammatory signals wired to the gut by the microbiota before altered structure and function in the target organ becomes consolidated (Fig. 1). The rationale is based on the model of a modern neonate, who is frequently exposed to unfavorable nutritional environment during pregnancy, born preterm or by cesarean section, or devoid of important immunomodulatory compounds of breast milk, and who thereby may lack an age-appropriate and environment-adjusted microbe contact. This in turn may substantially increase the child’s risk of developing noncommunicable diseases.
The Model Is the Healthy Breastfed Infant
The cornerstone of prevention of noncommunicable diseases is breastfeeding [23]. Not only does it provide the infant with nutrients, it may also confer im- munologic protection at the portal of entry where the major load of antigens is encountered, the gut barrier [24]. A delicate balance of stimulatory, even inflam- matory, maturational signals, together with a myriad of anti-inflammatory com- pounds, is transferred from mother to infant via breastfeeding. Human milk protective compounds also include specific oligosaccharides and fatty acids in- fluencing early microbial colonization and gut barrier adherence of pathogens and other microbes, but also specific microbiota and molecules operating in host-microbe interaction.
Infants who have been breastfed have lower infectious morbidity and mortality, and protection against noncommunicable diseases has also been implicated [23]. Medicalization of breastfeeding, however, is both unwarranted and unattainable. Breastfeeding is the model of infant feeding even if beyond the ultimate medical proof; any documentation of causality requires well-controlled randomized clinical intervention studies in different human populations, which cannot be completed to assess breast versus formula feeding modes for obvious ethical reasons. Moreover, observational long-term follow-up studies in breast- versus formula-fed infants also face one important confounder: the evolution of the formula composition. Today’s formula composition in energy and nonenergy nutrient composition strongly differs from that of past decades. The superiority of breastfeeding notwithstanding, infants who are exclusively breastfed may nevertheless develop allergic disease during breastfeeding or after weaning, or they develop other noncommunicable diseases later in life. This has been explained by the presence of antigens from the mother’s diet in breast milk or deficiency of key constituents in breast milk. In point of fact, the composition of the breast milk is not standard, but evinces marked individual variation, as a true product for personalized nutrition. The composition de- pends on environmental influences such as the mother’s immunological and metabolic health and the mode of delivery [25]. Moreover, early nutrition of the child needs to be considered when evaluating the long-term health effects of breastfeeding [26]. However, these comprise modifiable risk factors: the protective potential of breast milk and early nutrition can be enhanced as the science of diet, immune system, and microbiome interactions and the origins of human disease is evolving.
Determinants of Breast Milk Microbiota
The grounds for gut microbiota assembly may be generated already during the perinatal period. The mother provides the first inoculum of microbial colonization, possible already in utero (reviewed in the paragraph: Infant Gut Microbiota: Origin and Determinants of the Composition). There is abundant evidence that breast milk complements the microbiota transmission to the infant gut: the mother provides the infant with bifidobacteria, lactic acid bacteria, and other microbiota components in significant quantities during breastfeeding. Several active compounds of breast milk accomplish this progression. Among these, the breast milk microbiota profile is distinctive, reflecting the matrix of lipid and protein components. These components interact; the adhesion to mucosal surfaces, an important prerequisite of the immune-modulatory function of microbes, is modulated by polyunsaturated fatty acids.
Most of the bacteria isolated from breast milk belong to Staphylococcus and Streptococcus, followed by Lactobacillus and Bifidobacterium spp. [27]. Gut-as- sociated strictly anaerobic microbes belonging to Blautia, Clostridium, Collinsella, and Veillonella and also some butyrate-producing bacteria such as Coprococcus and Faecalibacterium, as well as Roseburia have also been isolated in breast milk [28]. However, the new sequencing methodologies have provided evidence of a rich and diverse breast milk microbial community [28].
Above health-promoting microbes, human milk provides their optimal growth factors, human milk oligosaccharides, comprising over 200 prebiotic oligosaccharide isomers. Oligosaccharides typically pass undigested from the infant stomach and are the major nutrient source available for the saccharolytic microbiota of the colon. Indeed, variation in the oligosaccharide profile in milk influences the microbial establishment in the infant gut. It has been reported that oligosaccharides favor the growth of specific gut bacterial groups such as Staphylococcus and Bifidobacterium spp. that also are present in breast milk [29]. The profile of human milk oligosaccharides has been described to affect infant gut microbial colonization by selectively promoting some bacteria and acting as decoy molecules for specific pathogens [30]. Infants fed by nonsecretor mothers, with lower presence of 2′FL (2′-fucosyllactose) exhibit delayed Bifidobacterium colonization and harbor lower numbers of Bifidobacterium than infants receiving breast milk from secretor mothers with higher abundance of 2′FL [31].
The microbes in breast milk strongly vary according to the mother’s health and weight gain during pregnancy [32]. To take an example, infants solely breastfed by their allergic and skin-prick-test-positive mothers had lower numbers of bifidobacteria than nonallergic mothers [33]. Similarly, Bacteroides and Staphylococcus were found to be significantly higher while Bifidobacterium counts were lower among gut microbes of overweight pregnant women and women with excessive weight gain during pregnancy than those with normal weight gain, and this distinction was reflected in the breast milk microbiota [32]. The mode of delivery has an influence on breast milk microbiota composi- tion; distinct profiles have been documented between mothers delivering vaginally compared to those undergoing cesarean section delivery, but also between the types of cesarean delivery, i.e., elective versus nonelective cesarean section [25, 34], pointing to an impact of the physiological labor process, stress, and/or hormonal signals on the microbiota composition. In general, higher microbial diversity and abundance of Bifidobacterium and Lactobacillus characterize the breast milk microbiota of mothers after vaginal deliveries as compared to those delivering by cesarean section, but not consistently in different populations [34–36].
Importantly, it appears that Bifidobacterium colonization frequencies and counts among mother-infant pairs correlate. Moreover, the impact of the gut microbiota on the mucosal immune system evolution is well documented as a strain-dependent property [37]. Taken together, the infant’s probability of being colonized by bifidobacteria is lower when the mother has a higher BMI, excessive weight gain during pregnancy, and the child is delivered via cesarean section, and higher when the mother is of normal weight, has noticeable bifidobacterial colonization in her own gut and breast milk, and is breastfeeding.
Bridging Early Nutrition to Health by the Microbiota
The gut microbiota holds a key position with regard to the increasing burden of noncommunicable diseases in the industrialized countries. Its composition is relevant to the risk of disease in the gastrointestinal tract: the interaction between microbes and mucosal innate and adaptive immune systems provide the basis for achieving a halt to the vicious circle of inflammation therein. Recent advances in clinical research have revealed that the gut microbiota has effects on host physiology and development also outside the gastrointestinal system.
Our intervention studies with long-term follow-up corroborate the fundamental value of the gut microbiota profile at an early age to later health [38–40]. The studies found that children later developing allergic manifestations or be- coming overweight had lower counts of bifidobacteria at the ages of both 6 and 12 months as compared to those remaining healthy, as well as a lower total Bifidobacterium genus pool, specifically of B. longum and B. breve. Moreover, administration of specific probiotics, compared to placebo, during the perinatal period and early infancy enabled to reduce the risk of allergic diseases through- out childhood until adolescence. Importantly, according to the multivariate logistic regression model, a lower risk of overweight was associated with breast- feeding duration ≥6 months compared to shorter duration.
Breastfeeding provides several health benefits [23] that are likely to be caused by promotion of age-appropriate and environment-adjusted gut colonization. Both precocious and delayed maturation of the gut microbiota seems to carry untoward immune and metabolic consequences [41]. It is of note that there are simultaneously occurring developments during infancy such as maturation of the gut barrier functions, reduction in breast milk consumption, and introduction of solid foods, which all impact on the compositional development of gut microbiota. While bifidobacteria typify the gut microbiota of a healthy breastfed infant, it is evident that Lactobacillaceae and Bifidobacteriaceae decrease upon introduction of solid foods and the transition period to family foods [reviewed in 21], with gut microbial diversity and richness significantly increasing concomitantly.
In general, the Western diet with its high fat and energy content has been associated with reduced gut microbiota diversity and perturbed composition, an imbalance in the taxonomic composition of the gut microbiota characterized as dysbiosis [42]. Reciprocally, the gut microbiota impacts on metabolism by retrieving nutrients otherwise inaccessible to the host; specific gut microbiota pro- files facilitate the extraction of calories from the diet and their storage in the host adipose tissue [43]. Additionally, active inflammatory cascades evolve reactive to a high-fat diet. Interestingly, dietary fatty acids and microbes engage the same signaling pathways, linking the nutritional environment to the gut microecology within the innate immune regulation [11, 44]. Specifically, increased Proteobacteria have been considered markers or “signatures” of intestinal dysbiosis [45] while Akkermansia muciniphila, a member of Verrucomicrobia, appears to correlate inversely with inflammation [46]. Based on an American study, the consumption of processed food was associated with a daily exposure to 106 bacteria as compared to a diet recommended by the dietary guidelines (109 bacteria) [47], thus demonstrating the decrease in richness.
The strong association between early nutrition and the compositional development of the gut microbiota, both impacting on the individual’s later health, invite the idea of next-generation personalized diets based on specific risk algorithms. These systems would certainly benefit from rapid diagnostics of the gut microbiota profiles. Indeed, it has been proposed that the gut microbiota provides the key determinant to be considered when developing specific dietary products for the need of both developing and developed countries [6, 41].
Taken together, dysbiosis is a necessary initial step in the development of noncommunicable diseases on the one hand and undernutrition on the other. An attractive vision arising from recent experimental and clinical studies is to identify and target disease risk by bringing the gut microbiota into balance. Reprogramming at an early age may necessitate well-adjusted age-appropriate food matrix for active compounds with scientifically proven safety and efficacy assessment, and the optimal timing of the intervention before consolidation of target organ dysfunction.
References
-
1 Levy M, Kolodziejcyk AA, Thaiss CA, Elinav E: Dysbiosis and the immune system. Nat Rev Immunol 2017;17:219–232.
-
2 Sevelsted A, Stokholm J, Bønnelykke K, Bis- gaard H: Cesarean section and chronic im- mune disorders. Pediatrics 2015;135:e92– e98.
-
3 Turta O, Rautava S: Antibiotics, obesity and the link to microbes – what are we doing to our children? BMC Med 2016;14:57.
-
4 Kau AL, Ahern PP, Griffin NW, et al: Human nutrition, the gut microbiome and the immune system. Nature 2011;474:327–336.
-
5 Moloney RD, Desbonnet L, Clarke G, et al: The microbiome: stress, health and disease. Mamm Genome 2014;25:49–74.
-
6 Rautava S, Luoto R, Salminen S, Isolauri E: Microbial contact during pregnancy, intestinal colonization and human disease. Nat Rev Gastroenterol Hepatol 2012;9:565–576.
-
7 Zhao L: The gut microbiota and obesity: from correlation to causality. Nat Rev Microbiol 2013:11:639–647.
-
8 Collado MC, Rautava S, Aakko J, et al: Hu- man gut colonization may be initiated in utero by distinct microbial communities in the placenta and amniotic fluid. Sci Rep 2016;6: 23129.
-
9 Bäckhed F, Roswall J, Peng Y, et al: Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe2015;17:690–703.
-
10 Dominguez-Bello MG, Costello EK, Contreras M, et al: Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci USA 2010;107:11971–11975.
-
11 Jakobsson HE, Abrahamsson TR, Jenmalm MC, et al: Decreased gut microbiota diversity, delayed Bacteroidetes colonisation and reduced Th1 responses in infants delivered by caesarean section. Gut 2014;63:559–566.
-
12 Penders J, Gerhold K, Stobberingh EE, et al: Establishment of the intestinal microbiota and its role for atopic dermatitis in early childhood. J Allergy Clin Immunol 2013;132: 601–607.
-
13 Endo A, Pärtty A, Kalliomäki M, et al: Long- term monitoring of the human intestinal microbiota from the 2nd week to 13 years of age.Anaerobe 2014;28:149–156.
-
14 Nogacka A, Salazar N, Suárez M, et al: Impact of intrapartum antimicrobial prophylaxis upon the intestinal microbiota and the prevalence of antibiotic resistance genes in vaginal- ly delivered full-term neonates. Microbiome 2017;5:93.
-
15 Azad MB, Konya T, Persaud RR, et al: Impact of maternal intrapartum antibiotics, method of birth and breastfeeding on gut microbiota during the first year of life: a prospective cohort study. BJOG 2016;123:983–993.
-
16 Tanaka S, Kobayashi T, Songjinda P, et al: Influence of antibiotic exposure in the early postnatal period on the development of intestinal microbiota. FEMS Immunol Med Microbiol 2009;56:80–87.
-
17 Fouhy F, Guinane CM, Hussey S, et al: High- throughput sequencing reveals the incomplete, short-term recovery of infant gut microbiota following parenteral antibiotic treatment with ampicillin and gentamicin. Antimicrob Agents Chemother 2012;56: 5811–5820.
-
18 Arboleya S, Sánchez B, Milani C, et al: Intestinal microbiota development in preterm neonates and effect of perinatal antibiotics. J Pediatr 2015;166:538–544.
-
19 Pannaraj PS, Li F, Cerini C, et al: Association between breast milk bacterial communities and establishment and development of the infant gut microbiome. JAMA Pediatr 2017; 171:647–654.
-
20 Perez PF, Dore J, Leclerc M, et al: Bacterial imprinting of the neonatal immune system: lessons from maternal cells? Pediatrics 2007; 119:e724–e732.
-
21 Laursen MF, Bahl MI, Michaelsen KF, Licht TR: First foods and gut microbes. Front Microbiol 2017;8:356.
-
22 Timmerman HM, Rutten NBMM, Boekhorst J, et al: Intestinal colonisation patterns in breastfed and formula-fed infants during the first 12 weeks of life reveal sequential microbiota signatures. Sci Rep 2017;7:8327.
-
23 Victora CG, Bahl R, Barros AJ, et al: Breast- feeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet 2016; 387:475–490.
-
24 Donovan SM, Comstock SS: Human milk oligosaccharides influence neonatal mucosal and systemic immunity. Ann Nutr Metab 2016;69(suppl 2):42–51.
-
25 Cabrera-Rubio R, Collado MC, Laitinen K, et al: The human milk microbiome changes over lactation and is shaped by maternal weight and mode of delivery. Am J Clin Nutr 2012;96:544–551.
-
26 Peneau S, Hercberg S, Rolland-Cachera M-F: Breast-feeding, early nutrition, and adult body fat. J Pediatr 2014;164:1363–1368.
-
27 Fitzstevens JL, Smith KC, Hagadorn JI, et al: Systematic review of the human milk microbiota. Nutr Clin Pract 2017;32:354–364.
-
28 Gomez-Gallego C, Garcia-Mantrana I, Sal- minen S, Collado MC: The human milk microbiome and factors influencing its compo- sition and activity. Semin Fetal Neonatal Med 2016;21:400–405.
-
29 Aakko J, Kumar H, Rautava S, et al: Human milk oligosaccharide categories define the microbiota composition in human colostrum. Benef Microbes 2017;8:563–567.
-
30 Wang M, Li M, Wu S, et al: Fecal microbiota composition of breast-fed infants is correlated with human milk oligosaccharides consumed. J Pediatr Gastroenterol Nutr 2015;60:825–833.
-
31 Lewis ZT, Totten SM, Smilowitz JT, et al: Maternal fucosyltransferase 2 status affects the gut bifidobacterial communities of breastfed infants. Microbiome 2015;3:13.
-
32 Collado MC, Isolauri E, Laitinen K, Salminen S: Distinct composition of gut microbiota during pregnancy in overweight and normal weight women. Am J Clin Nutr 2008;88:894– 899.
-
33 Grönlund M-M, Gueimonde M, Laitinen K, et al: Maternal breast-milk and intestinal bifidobacteria guide the compositional development of the Bifidobacterium microbiota in infants at risk of allergic disease. Clin Exp Allergy 2007;37:1764–1772.
-
34 Cabrera-Rubio R, Mira-Pascual L, Mira A, Collado MC: Impact of mode of delivery on the milk microbiota composition of healthy women. J Dev Orig Health Dis 2016;7:54–60.
-
35 Urbaniak C, Angelini M, Gloor GB, Reid G: Human milk microbiota profiles in relation to birthing method, gestation and infant gender. Microbiome 2016;4:1.
-
36 Sakwinska O, Moine D, Delley M, et al: Microbiota in breast milk of Chinese lactating mothers. PLoS One 2016;11:e0160856.
-
37 He F, Morita H, Hashimoto H, et al: Intestinal Bifidobacterium species induce varying cytokine production. J Allergy Clin Immunol 2002;109:1035–1036.
-
38 Kalliomäki M, Kirjavainen P, Eerola E, et al: Distinct patterns of neonatal gut microflora in infants in whom atopy was and was not developing. J Allergy Clin Immunol 2001; 107:129–134.
-
39 Kalliomäki M, Collado MC, Salminen S, Isolauri E: Early differences in fecal microbiota composition in children may predict over- weight. Am J Clin Nutr 2008;87:534–538.
-
40 Lundelin K, Poussa T, Salminen S, Isolauri E: Long-term safety and efficacy of perinatal probiotic intervention: evidence from a fol- low-up study of four randomized, double- blind, placebo-controlled trials. Pediatr Allergy Immunol 2017;28:170–175.
-
41 Subramanian S, Blanton LV, Frese SA, et al: Cultivating healthy growth and nutrition through the gut microbiota. Cell 2015;161: 36–48.
-
42 Hold GL: Western lifestyle: a “master” manipulator of the intestinal microbiota? Gut 2014;63:5–6.
-
43 Bäckhed F, Ding H, Wang T, et al: The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA 2004;101:15718–15723.
-
44 Laitinen K, Poussa T, Isolauri E: Probiotics and dietary counselling contribute to glucose regulation during and after pregnancy: randomized clinical trial. Br J Nutr 2009;101: 1679–1687.
-
45 Shin NR, Whon TW, Bae JW: Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol 2015;33:496–503.
-
46 Schneeberger M, Everard A, Gómez-Valadés AG, et al: Akkermansia muciniphila inversely correlates with the onset of inflammation, altered adipose tissue metabolism and metabolic disorders during obesity in mice. Sci Rep 2015;5:16643.
-
47 Lang JM, Eisen JA, Zivkovic AM: The microbes we eat: abundance and taxonomy of microbes consumed in a day’s worth of meals for three diet types. Peer J 2014;2:e659.