Early-Life Nutrition and Gut Immune Development
Abstract
Gut immune function conditions the development of local and systemic diseases that result from defects in immune regulation, such as inflammatory bowel disease, allergy and obesity. As epidemiological studies support the developmental origin of health and disease, deciphering the critical factors modulating gut immune development should allow the advance of primary prevention strategies specifically adapted to the early-life immune system. Here, we will review gut mucosal immunity development and cover in more detail the recent understanding of the impact of early nutrition on this process. We will emphasize how nutrition can shape microbiota composition and metabolic function and thereby the production of metabolites with immune-modulatory properties. We will also focus on the role of dietary compounds recently demonstrated to be essential in immune development and function, such as dietary antigens, vitamin A, and aryl hydrocarbon receptor ligands. Finally, we will discuss that early-life physiologic food for mammals contains factors capable of compensating for neonatal immune deficiencies, but also factors that are decisive for immune maturation towards a maternal milk-independent and efficient immune system.
Introduction
Early-life gut immune function can be qualified as deficient according to the high susceptibility of neonates and infants to enteric infections. The high incidence of food allergy in infants further highlights a deficient capacity to mount oral tolerance. Mucosal immunity will mature in postnatal life to evolve, ideally, to mount regulatory immune responses towards dietary and nonpathogenic microbial antigens and inflammatory responses against pathogens. In parallel to immune system maturation, the gut develops from a nearly sterile environment at birth to harboring a microbiota that is very simple and highly variable and eventually reaches a dense, complex, and stable microbiota around 3 years of life [1]. Both epidemiological and experimental studies have linked perturbations in gut microbiota composition in early life with the risk of developing immune- mediated disease in later life [reviewed in 2]. Infants at risk of asthma show a reduction in the relative abundance of certain bacterial genera during their first 3 months of life, while no major difference in microbiota composition was found at older ages. Clinical studies also suggest that disturbances in the intestinal flora early in life, caused by cesarean section delivery or early antibiotic exposure, may contribute to the development of diseases such as food allergy, inflammatory bowel disease, and types 1 and 2 diabetes [2].
Here, we will review experimental evidence that nutrition can affect gut immune ontogeny directly and indirectly by shaping microbiota composition, with lifelong impact on immune homeostasis.
Gut Immune Ontogeny
The gut epithelium progresses from being flat and poorly proliferating towards a highly proliferative epithelium, with crypt and villus architecture dramatically expanding the absorptive surface. This development is completed at birth in humans while still developing postnatally in mice [reviewed in 3]. A high gut per- meability is found in the neonate, both in mice and humans. In mice, few goblet and Paneth cells are present at birth and there is a low secretion of IgA in the gut lumen. Neonatal enterocytes however produce CRAMP (cathelicidin-related antimicrobial peptide) that acts as an antimicrobial defense mechanism specific to the early-life period, as it is also found in breast milk and the vernix caseosa. The high susceptibility of preterm neonates to necrotizing enterocolitis shows that an immature gut epithelium is susceptible to highly inflammatory respons- es; however, regulatory mechanisms such as decreased Toll-like receptor (TLR), TLR3 and TLR4, expression and signaling are found in neonates born at term, which contribute to dampening inflammatory responses upon microbial colonization [3].
Peyer’s patches and mesenteric lymph nodes (MLNs) develop in utero while isolated lymphoid follicles (ILF) do so after birth [4]. A common scheme operates for lymphoid tissue formation: a stromal cell produces chemokines (CXCL13) which will attract CXCR5-expressing lymphoid tissue inducer (LTi) cells of hematopoietic origin (more recently known as a subset of innate lymphoid cells [ILC], ILC3, expressing RAR-related orphan receptor γ [RORγ]t). Binding of lymphotoxin (LT) αβ secreted by LTi to LTβ receptors expressed by stromal cells induces chemokine secretion and adhesion molecule expression needed for the attraction and retention of additional hematopoietic cells, leading to lymph node growth [4]. Peyer’s patches require CD11c+ cells expressing the receptor tyrosine kinase RET, in addition to LTi and stromal cells, for their for- mation. ILF originate from cryptopatches, which are a cluster of LTi in the lamina propria. These expand after birth in the presence of B cells and a few T cells to become ILF [4].
A comprehensive analysis of the emergence of immune cells in murine neonatal small intestine was recently undertaken [reviewed in 3]. This showed that myeloid cells are found in the lamina propria at birth, and their numbers remain stable postnatally. We further found that, although in similar frequency in neonatal MLNs as compared to the adult, the capacity of neonatal CD103+ dendritic cells (DCs) to metabolize retinol was significantly impaired [5]. Importantly, we found this defect responsible for inefficient oral tolerance induction in the neonate [5]. After a massive recruitment of CD4+ T cells and B lymphocytes into the gut mucosa during the first 2 days of life, B cells were found to continue to expand, while TCR-αβ lymphocytes did so only after weaning. To- row and Hornef [3] also found that, before weaning, CD4+ T lymphocytes were mostly found in Peyer’s patches, leaving the lamina propria relatively empty compared to the adult. They further uncovered that these lymphocytes exhibit a naïve phenotype until weaning, due to active suppression by FoxP3+ regulatory T cells (Tregs) and maternal milk IgA. Similar to mice, a recent analysis of T-cell representation and differentiation in tissues of infants from 2 months to 2 years of age revealed a higher proportion of naïve and regulatory T cells in all tissues compared to the adult [6]. Except for the subset of ILC3 involved in lymphoid tissue morphogenesis, LTi, limited data are currently available addressing the representation and function of ILCs in neonatal gut mucosa.
In summary, early postnatal life gut mucosal immunity is characterized by a leaky barrier with poorly developed innate and adaptative effector mechanisms, which are kept under control by regulatory cells. Breastfeeding is therefore critical for the prevention of infectious diseases in young infants as it provides the breastfed infant with antigen-specific (IgA and IgG) and nonantigen-specific antimicrobial molecules (lysozyme, lactoferrin, oligosaccharides, and leukocytes) [7]. Despite the high proportion of Tregs in the neonatal gut mucosa, oral tolerance can hardly be induced in neonates [reviewed in 7], and highly inflammatory reactionssuchas necrotizing enterocolitis cantake place intheneonatal gut. Here also, breast milk compensates for the lack of molecules involved in immunoregulation in the neonatal gut. TGF-β, epidermal growth factor, human milk oligosaccharides (HMOs), and maternal IgG and IgA present in breast milk were in- deed found to exert anti-inflammatory and immunoregulatory effects [7, 8]. In the next parts of this review, we will analyze how nutrition is involved in neonatal gut immune maturation towards an autonomous system, no longer dependent on breast milk to mount efficient regulatory and effector immune responses.
Impact of Nutrition on Gut Mucosal Immune Ontogeny through Microbiota Shaping
Gut Microbiota Ontogeny
Besides the mode of delivery, known to strongly affect colonization of the neonatal intestine within the first days after birth, nutrition is the key factor directing the early microbiota composition and function [reviewed in 1]. The gut flora in breastfed infants is usually dominated by Bifidobacterium and Lactobacillus species, while formula-fed infants harbor a more diverse gut microbiota with increased abundance of Escherichia coli, Clostridium, and Bacteroides. The microbiomes of newborns and young infants are enriched in genes required for the degradation of sugars from breast milk, such as HMOs. Upon weaning, the microbiota functionally maturates by a decrease in the relative abundance of genes involved in the degradation of HMO and enrichment of genes involved in the degradation of complex sugars and starch. Cessation of breastfeeding, not solid food introduction, is critical for this shift.
Mechanisms of Microbiota–Driven Immune Shaping
Germ-free (GF) mice represent an extreme situation that illustrates the necessity of microbiota for gut immune development. Gut microbiota was shown to be necessary for ILF formation in the small intestine and the development of MLN and Peyer’s patches. Differentiation of immune responses is also dependent on microbial colonization. Microbiota is necessary for IgA secretion, regulation of IgE responses, differentiation of Th17 and Th1 cells, and RORγt+ Treg expansion in the colon [9]. Gut microbial composition exerts direct effects on the immune system through microorganism-associated molecular pattern (MAMP) signaling and indirectly via the production of metabolites. An example of a well-studied MAMP is polysaccharide A from Bacteroides fragilis that was found to promote Treg differentiation and MYD88-signaling involved in epithelium repair and antimicrobial peptide secretion [9]. Fermentation of dietary fibers in the colon by anaerobic bacteria, such as clostridia and bifidobacteria, generate short-chain fatty acids (SCFAs) including butyrate, acetate, and propionate. SCFAs signal through G-protein-coupled receptors, such as GPR43, GPR41, and GPR109A, present on epithelial and immune cells, and via inhibition of histone deacetylases, with long-term consequences through epigenetic modification. Among many of their reported immune effects, SCFAs promote colonic Treg differentiation, mucus production, and IgA secretion (Fig. 1a). Commensals, particularly Lactobacillus, metabolize tryptophan, an essential amino acid that is a common constituent of protein-based foods such as eggs, fish, meat, and cheese (Fig. 1b). The metabolites, which are indole derivatives, bind aryl hydrocarbon receptor (AhR). AhR was found to be necessary for ILC3 function both in postnatal development of ILF and IL-22 secretion necessary for gut barrier function and protection from Citrobacter infection and colitis [9].
While most of the studies addressing the role of the gut microbiota on immune maturation have been performed in adults and observations extrapolated to the developing neonate, some recent publications have specifically addressed the impact of colonization in early life on immune ontogeny [reviewed in 2]. These have started to show clear specificities of early-life immune responses to microbiota colonization and highlight the concept of a window of opportunity to induce a lifelong effect on immune homeostasis by shaping the microbiota in early life. Thus, GF mice conventionalized during adult life exhibit a different transcriptional profile in jejunum and colon compared to conventionally raised mice. High IgE found in GF mice can only be normalized if colonization occurs before 4 weeks of age. Another detailed mechanistic study showed that colonization of GF mice with gut microbiota in the first 2 weeks of life, but not in adults, was sufficient to protect mice from increased susceptibility to colitis due to mucosal invariant natural killer T (iNKT) cell accumulation. The protective effect of colonization in early life could also be induced by B. fragilis monocolonization and depends on polysaccharide A signaling. Colonization with Clostridium during weaning in- duces colonic Treg and fecal IgA as well as IL-22 production by RORγt+ ILCs and T lymphocytes, promoting gut barrier function and resistance to food allergy [10]. In contrast, neonatal colonization with specific strains of the commensal E. coli impairs oral tolerance induction by affecting intestinal permeability and the balance of tolerogenic DCs and Tregs through the production of a genotoxin [11].
Age-specific mechanisms of action of microbiota-driven immune shaping were also found in neonates. Gomez de Aguero et al. [12] found that maternal microbiota generates AhR-binding metabolites that are transferred in utero and postnatally through breast milk and induce mononuclear cells and ILC3 expansion. The latter effect was found to be increased by maternal antibodies in the milk. The strong impact of gut microbiota on immune function has stimulated research on the potential to promote infant microbiota development by oral ad- ministration of probiotics to induce health benefits. Despite an increase in probiotic administration, data supporting their efficacy is lacking [1]. Another strategy is to promote the growth of beneficial bacteria using prebiotics.
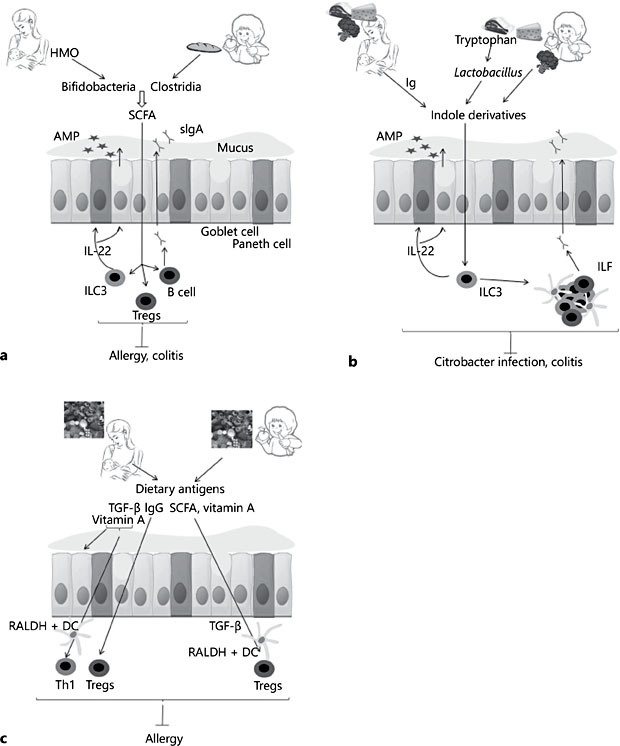
Fig. 1. Impact of food on immune ontogeny. a Short-chain fatty acids (SCFAs) stimulate regulatory T-cell expansion, IgA, and mucus secretion, gut epithelium barrier function, an- timicrobial peptide secretion (AMP), and innate lymphoid cell (ILC)3 function and induce resistance to food allergy and gut inflammatory disease. Human milk oligosaccharides (HMO) are present in human milk and stimulate the growth of bifidobacteria that can me- tabolize HMO into SCFA. After weaning, the metabolic function of bifidobacteria changes, and they become capable of metabolizing complex sugars from dietary fibers, similarly to clostridia found in microbiota of older children. b Aryl hydrocarbon receptor (AhR) ligands bind to AhR receptor expressed on ILC3. They stimulate the postnatal formation of isolated lymphoid follicles (ILF) and IL-22 secretion necessary for gut barrier function and protec- tion from Citrobacter infection and colitis. AhR ligands are found in cruciferous vegetables such as broccoli and cabbage. They can also be produced by some commensals, such as Lactobacillus, which metabolize tryptophan from protein-based foods into indole deriva- tives. In breastfed infants, AhR ligandscan originate from maternal microbiota metabolites with maternal milk immunoglobulin helping in the transfer of these metabolites to the neonate. Breast milk may also contributes to AhR-mediated immune ontogeny by stimu- lating the growth of Lactobacillus. c After weaning, antigens derived from solid food are necessary to populate the small intestine with induced Tregs. Tregs specific to dietary an- tigens can be induced by oral exposure. Before weaning, oral tolerance can be induced to antigens from the maternal diet which are present in breast milk. This requires the pres- ence of additional cofactors in breast milk such as TGF-β, vitamin A, and IgG. Vitamin A in- creases gut barrier function, the capacity of dendritic cells (DCs) to metabolize vitamin A into retinoic acid and Th1 differentiation. Antigens bound to IgG are better transported across the epithelium and induce FoxP3 Tregs that are responsible for potent and long- lasting tolerance. After weaning, Treg induction towards oral antigen is favored by SCFAs. These induce TGF-β secretion from epithelium and stimulate retinoic acid formation from vitamin A by DCs.
Early Nutrition Driving Microbiota Composition and Function
Breast milk contains 102 to 104 viable bacteria/mL that will directly affect the establishment of the neonatal microbiota. It also contains prebiotic and immunologic compounds that can alter colonization patterns in the neonate. In particular, HMOs stimulate the growth of bifidobacteria, which metabolize these oligosaccharides into SCFAs that favor immune regulation (Fig. 1a). HMO also have the capacity to modify the gene expression involved in metabolic function in commensals and thereby their secretion of metabolites which can affect growth [13] and inflammation [14]. Metabolized HMOs are also beneficial for other commensals that do not directly degrade HMOs [13]. In this context, dietary and synthetic oligosaccharides are the object of studies to assess similar early-life immune regulation, with promising results [1]. Breast milk secretory IgA antibodies are specific for an array of common intestinal pathogens and commensals due to the selective migration of B cells originating from the mucosal membranes to the mammary gland. In addition to providing excellent passive mucosal immunity to neonates, maternal IgA and other molecules found in breast milk with antimicrobial properties, such as lactoferrin and lysozyme, will shape the microbiota composition of the breastfed infant [7].
Direct Impact of Nutrition on Gut Mucosal Immune Ontogeny
Here, we will focus on the impact of breastmilk and selected nutrients that have recently been the focus of experimental studies in early life for their impact on immune ontogeny, that is, dietary antigens, vitamin A, RORγt, and AhR ligands.
Solid Food and Dietary Antigens
The role of dietary antigens on gut mucosal immunity ontogeny has been assessed by comparing mice that were free of specific pathogens, that were devoid of microbiota (GF mice), or that were devoid of both microbiota and dietary- derived antigen (antigen-free mice) from birth [15]. Others also studied the impact of dietary antigen after weaning on immune ontogeny of specific pathogen- free mice [16]. These studies demonstrate that, after weaning, dietary-derived antigens are necessary and sufficient to stimulate memory CD4+ T lymphocytes and peripherally induced Tregs to populate small-intestine lamina propria. They are required for controlling the Th2 immune response and susceptibility to food allergy and necessary for IgA and IgG secretion. Evidence that exposure to diet-derived proteins is important to induce immune regulation also arises from intervention studies analyzing the impact of food diversification in the first year of life [17]. These observations, made both in mice and in humans, high- light that one should consider the administration of an extensively hydrolyzed formula to infants with allergies with care, as this nutritional approach may decrease regulatory function of the gut.
The shaping of immune reactivity by induction of oral tolerance to specific antigens during the period of immune ontogeny, was recently reviewed [17]. Egg introduction between 4 and 6 months was able to prevent from egg allergy, and peanut introduction between 4 and 11 months was able to prevent from peanut allergy [17]. However, the results obtained for the prevention of peanut allergy required adherence to the protocol that would hardly be achievable in the daily life [17]. Furthermore, the early introduction of other allergens such as fish or milk did not prevent allergy, and early introduction of gluten did not reduce the risk of celiac disease [17]. Overall, these data show that promoting tolerance by oral antigen exposure during immune ontogeny is possible, but that additional cofactors are required to enhance the chance of success. In this regard, the role of breast milk factors in promoting oral tolerance has attracted increased interest. Antigens from the maternal diet are found in breast milk at concentrations 1,000-fold lower (ng/ mL) than antigen levels in formula milk (mg/mL). Unexpectedly, we also found antigens of respiratory sources such as the house dust mite Dermatophagoides pteronyssinus (Der p) and Blomia tropicalis in breast milk, in similar amounts as dietary antigen [18, 19]. Since we detected Der p 1 in digestive fluid of healthy adults [20], we propose that respiratory allergens are ingested by being trapped in the oropharynx or pulled back by the mucociliary epithelium and follow the same route as dietary antigens to the mammary gland. We have specifically addressed the factors in breast milk that could improve the chance of oral tolerance induction to dietary and respiratory antigens in rodents. We found that mice exposed to a few nanograms of egg ovalbumin (OVA) antigen through breast milk were protected from OVA-induced allergic airway disease and food allergy [21, 22]. Importantly, TGF-β from breast milk was necessary for oral tolerance induction [22]. We demonstrated protection to be more profound when OVA was transferred through the breast milk of OVA-immunized mothers than OVA-exposed nonimmunized mothers [23]. OVA-specific IgG in milk was necessary for protection during transfer of OVA though the gut barrier and the induction of a pro- longed protection mediated by FoxP3 Tregs. We also identified a key role of vita- min A in breast milk in the process of neonatal gut immunity maturation (see below) [5]. Our more recent data further indicated that the nature of antigen found in breast milk could dramatically affect immune outcome. In contrast to the observation with OVA, the transfer of Der p 1 through breast milk induced Th2 immune response priming and increased susceptibility to allergic disease in adult mice [24]. Importantly, in a human birth cohort, the risk of allergic sensitization and respiratory allergies in children breastfed by mothers increased with Der p 1 levels in breast milk [18]. This observation stresses that not all the antigens in breast milk induce oral tolerance, and that there is a need to identify how maternal milk factors could be modulated to counteract the deleterious actions of some allergens. Ongoing intervention studies will help to decipher whether antigens in breast milk impact on immune tolerance induction [25].
Vitamin A
Vitamin A is found in animal-derived food such as milk, liver, and egg yolk, while its precursors, the carotenoids, are found in vegetables such as carrots and broccoli. Major advances have recently been made on the impact of vitamin A and its metabolite retinoic acid (RA) on immune homeostasis [7]. Specifically, RA pro- motes CD4+ T cell differentiation, supports the generation of IgA-secreting B cells, and mediates the balance between ILC3 and ILC2. RA also imprints gut- homing specificity on T and B cells to the small intestine. Vitamin A in conjunction with SCFA derived from fibers metabolized by microbiota, promoted oral tolerance and prevented food allergy [26]. We recently identified that neonatal mice are physiologically deficient in retinol; serum retinol levels then progressively increase and reach adult levels at 3 weeks due to breast milk vitamin A [5]. Low vitamin A levels at birth were found to be responsible for a leaky gut barrier, deficient RALDH expression by MLN CD103+ neonatal DCs, resulting in inefficient T-cell activation and the incapacity to induce oral tolerance in neonates [5]. Importantly, vitamin A supplementation was sufficient to accelerate gut epithelium differentiation in terms of architecture and barrier function while preserving the capacity of epithelial cells to digest milk sugars [5]. It also promoted immune maturation and allowed tolerance induction from birth, as observed in 3-week-old mice. Our observations also showed that vitamin A was involved in the maturation of the neonate’s immune responses towards Th1 immunity; this adds a dietary factor to the genetically programmed and microbiota-driven neonatal Th1 immune maturation [5]. Relevance of these data for the human is supported by reports on low retinol levels in healthy infants from well-nourished countries [5] and observational studies linking low retinol levels at birth with increased atopic risk in young adults [27]. In early postnatal life, vitamin A may also be involved in immune ontogeny by acting on lymphoid tissue organogenesis and development. Indeed, RA was shown to be necessary both for CXCL13 secretion by stromal cells [4], the first step in lymphoid tissue organogenesis, and for LTi differentiation and lymph node development [28]. This points to an important role of maternal intake of vitamin A and precursors during pregnancy in immune ontogeny and possibly in early postnatal life for lymph node development.
Aryl Hydrocarbon Receptor and RAR-Related Orphan Receptor-γt Ligands
RORγt is a master transcription factor for the development of lymphoid organs, Th17, and ILC3. Their presence in GF animals suggests that the microbiota is not a critical source of RORγt ligand [29]. The natural ligand was recently identified as a derivative of cholesterol, indicating that sterol metabolism may be essential for proper lymphoid tissue development in utero and possibly in early postnatal life for ILF.
In addition to binding microbiota-derived metabolites of tryptophan and pollutants, AhR binds dietary ligands contained in cruciferous vegetables, such as broccoli, that may then impact on gut immunity development after weaning. During the lactation period, AhR ligands in breast milk could originate from the maternal diet as well as from maternal microbiota-derived metabolites [12].
Breast Milk
Before weaning, breast milk supplies the neonate with antimicrobial and regulatory factors that complement its developing immune system. Breast milk is also providing the neonate with the factors necessary for immune maturation that the neonatal mammal would otherwise miss due to the lack of microbiota and solid-food-derived molecules. The maturating impact of breast milk was high- lighted in a recent analysis of exfoliated gut epithelial cells in stools of 3-month- old children that were breastfed versus those formula fed and that showed a total of 1,214 genes differentially expressed between breastfed and formula-fed children [30]. Analysis of gene networks reflected broad differences with respect to signal transduction, cytoskeletal remodeling, cell adhesion, and immune response. Gut trophic factors such as epidermal growth factor, HMOs, and vita- min A found in human milk are most probably involved in these effects [7]. Breast milk exposes the infant to a variety of food antigens, and it contains ligands that are critical for lymphoid tissue development and immune function such as AhR ligands and vitamin A. It provides HMOs, as surrogates to fibers found in solid food, for commensals to produce SCFAs. Breast milk also delivers microbiota and food for commensal growth in the sterile neonate gut.
Conclusion
While the impact of gut microbiota-derived antigens and metabolites on gut mucosal immunity has largely been demonstrated, there is growing experimental and clinical evidence that diet may be as important for immune ontogeny and function. Before weaning, maternal milk, the physiological food for mammals, will reassemble all the varied exogenous factors required for immune maturation. Solid-food-derived antigens, vitamins, lipids, as well as food metabolites produced by the microbiota will then continue to shape the immune system function and dictate long-term susceptibility to local and systemic immune- mediated disease.
References
- 1 van Best N, Hornef MW, Savelkoul PH, Penders J: On the origin of species: factors shaping the establishment of infant’s gut microbiota. Birth Defects Res C Embryo Today 2015;105:240–251.
- 2 Gensollen T, Iyer SS, Kasper DL, Blumberg RS: How colonization by microbiota in early life shapes the immune system. Science 2016; 352:539–544.
- 3 Torow N, Hornef MW: The neonatal window of opportunity: setting the stage for life-long host-microbial interaction and immune homeostasis. J Immunol 2017;198:557–563.
- 4 van de Pavert SA, Mebius RE: New insights into the development of lymphoid tissues. Nat Rev Immunol 2010;10:664–674.
- 5 Turfkruyer M, Rekima A, Macchiaverni P, et al: Oral tolerance is inefficient in neonatal mice due to a physiological vitamin A deficiency. Mucosal Immunol 2016;9:479–491.
- 6 Thome JJ, Bickham KL, Ohmura Y, et al: Ear- ly-life compartmentalization of human T cell differentiation and regulatory function in mucosal and lymphoid tissues. Nat Med 2016;22:72–77.
- 7 Turfkruyer M, Verhasselt V: Breast milk and its impact on maturation of the neonatal immune system. Curr Opin Infect Dis 2015;28: 199–206.
- 8 Koch MA, Reiner GL, Lugo KA, et al: Maternal IgG and IgA antibodies dampen mucosal T helper cell responses in early life. Cell 2016; 165:827–841.
- 9 Rooks MG, Garrett WS: Gut microbiota, metabolites and host immunity. Nat Rev Immunol 2016;16:341–352.
- 10 Stefka AT, Feehley T, Tripathi P, et al: Commensal bacteria protect against food allergen sensitization. Proc Natl Acad Sci USA 2014; 111:13145–13150.
- 11 Secher T, Payros D, Brehin C, et al: Oral tolerance failure upon neonatal gut colonization with Escherichia coli producing the genotoxin colibactin. Infect Immun 2015;83:2420–2429.
- 12 Gomez de Aguero M, Ganal-Vonarburg SC, Fuhrer T, et al: The maternal microbiota drives early postnatal innate immune development. Science 2016;351:1296–1302.
- 13 Charbonneau MR, O’Donnell D, Blanton LV, et al: Sialylated milk oligosaccharides promote microbiota-dependent growth in models of infant undernutrition. Cell 2016;164: 859–871.
- 14 Gonzalez R, Klaassens ES, Malinen E, et al: Differential transcriptional response of Bifidobacterium longum to human milk, formula milk, and galactooligosaccharide. Appl Environ Microbiol 2008;74:4686–4694.
- 15 Kim KS, Hong SW, Han D, et al: Dietary antigens limit mucosal immunity by inducing regulatory T cells in the small intestine. Science 2016;351:858–863.
- 16 Menezes JS, Mucida DS, Cara DC, et al: Stimulation by food proteins plays a critical role in the maturation of the immune system. Int Immunol 2003;15:447–455.
- 17 Palmer DJ, Prescott SL, Perkin MR: Early introduction of food reduces food allergy – pro and con. Pediatr Allergy Immunol 2017;28: 214–221.
- 18 Baiz N, Macchiaverni P, Tulic MK, et al: Early oral exposure to house dust mite allergen through breast milk: a potential risk factor for allergic sensitization and respiratory allergies in children. J Allergy Clin Immunol 2017;139:369–372.e10.
- 19 Macchiaverni P, Ynoue LH, Arslanian C, et al: Early exposure to respiratory allergens by placental transfer and breastfeeding. PLoS One 2015;10:e0139064.
- 20 Tulic MK, Vivinus-Nebot M, Rekima A, et al: Presence of commensal house dust mite allergen in human gastrointestinal tract: a potential contributor to intestinal barrier dysfunction. Gut 2016;65:757–766.
- 21 Rekima A, Macchiaverni P, Turfkruyer M, et al: Long-term reduction in food allergy susceptibility in mice by combining breastfeeding-induced tolerance and TGF-β-enriched formula after weaning. Clin Exp Allergy 2017;47:565–576.
- 22 Verhasselt V, Milcent V, Cazareth J, et al: Breast milk-mediated transfer of an antigen induces tolerance and protection from allergic asthma. Nat Med 2008;14:170–175.
- 23 Mosconi E, Rekima A, Seitz-Polski B, et al: Breast milk immune complexes are potent inducers of oral tolerance in neonates and prevent asthma development. Mucosal Immunol 2010;3:461–474.
- 24 Macchiaverni P, Rekima A, Turfkruyer M, et al: Respiratory allergen from house dust mite is present in human milk and primes for allergic sensitization in a mouse model of asthma. Allergy 2014;69:395–398.
- 25 Venter C, Brown KR, Maslin K, Palmer DJ: Maternal dietary intake in pregnancy and lactation and allergic disease outcomes in off- spring. Pediatr Allergy Immunol 2017;28: 135–143.
- 26 Tan J, McKenzie C, Vuillermin PJ, et al: Dietary fiber and bacterial SCFA enhance oral tolerance and protect against food allergy through diverse cellular pathways. Cell Rep 2016;15:2809–2824.
- 27 Pesonen M, Kallio MJ, Siimes MA, Ranki A: Retinol concentrations after birth are inversely associated with atopic manifestations in children and young adults. Clin Exp Allergy 2007;37:54–61.
- 28 van de Pavert SA, Ferreira M, Domingues RG, et al: Maternal retinoids control type 3 innate lymphoid cells and set the offspring immunity. Nature 2014;508:123–127.
- 29 Santori FR, Huang P, van de Pavert SA, et al: Identification of natural RORγ ligands that regulate the development of lymphoid cells. Cell Metab 2015;21:286–297.
- 30 Chapkin RS, Zhao C, Ivanov I, et al: Noninvasive stool-based detection of infant gastrointestinal development using gene expression profiles from exfoliated epithelial cells. Am J Physiol Gastrointest Liver Physiol 2010; 298:G582–G589.