Early-Life Nutrition and Cognitive Development: Imaging Approaches
Abstract
Brain development in the first years of life is the most dynamic and perhaps the most important phase of brain maturation. While it is widely recognized that nutrition plays a key role in early brain development, particular nutrients will most likely differentially affect distinct aspects of brain development. The critical dosage windows and time frames for various nutrients at different stages of brain development are likely dissimilar. There- fore, efforts have been devoted to identifying potential associations between nutrients and early brain development. However, behavioral assessments are typically employed as the outcome measures, which are known to suffer from low sensitivity and the inability to provide neural substrates underlying brain functional maturation. In contrast, magnetic resonance imaging is capable of providing detailed anatomical and functional in- formation – an ideal tool to characterize brain functional development and nutrition. Our team has developed strategies that enable imaging of typically developing children from birth to teens without sedation. Quantitative assessments of brain structural and functional development during the first years of life have been accomplished, which reveal important features of early brain development. These developed tools will most likely substantially enhance our ability to rigorously characterize the interplay between nutrients and early brain development.
Introduction
Brain development in the first years of life is the most dynamic and perhaps the most important phase of brain functional maturation [1]. Thus, efforts to shed light on the underlying maturation processes have been actively pursued [2–5]. Early brain development can potentially be categorized into 7 cellular stages encompassing both pre- and postnatal periods [6]. Neurogenesis represents the first stage of brain development, followed by cell migration (2nd stage). Once neurons arrive at their final destinations, cell differentiation occurs (3rd stage). While it has been shown that neurogenesis, cell migration, and cell differentiation are also present in a selected few brain regions postnatally [7], they predominately occur prior to birth. In contrast, the remaining 4 stag- es – dendrite and axonal growth, synaptogenesis, synaptic pruning, and my- elination – mainly arise postnatally. These complex and dynamic cellular processes set the foundation for remarkable cognitive maturation processes during the first years of life. Basic sensory functions such as visual and auditory functions start to develop during the third trimester and continue throughout the first years of life [8]. Language functions emerge during the second half of the first year and continue to mature throughout the first several years of life. Al- though higher-order brain functions are known to mature later in life and fol- low a protracted developmental trajectory, primitive higher-order brain functional networks resembling those observed in adults have also been reported during the first year of life [9, 10]. In addition, joint attention emerges around 9–10 months [11], which includes a set of complex behaviors considered as necessary precursors for (1) subsequent social communication such as the acquisition of spoken language and (2) increasingly complex social-cognitive capacities such as Theory of Mind [12]. Working memory capacity rapidly increases during this interval [13], laying the foundation for the development of more complex executive functioning. Infants between 9 and 10 months of age also begin to demonstrate more specialized face-processing skills (e.g., losing the global ability to effectively/consistently discriminate faces from other species) and specialized language processing (e.g., losing the global ability to discriminate nonnative language phonemes). Although basic brain functions, such as fine motor skills and auditory function, continue to mature, emphases on maturation of higher-order brain functions emerge rapidly after the first years of life and persist to teens.
Any adverse effects leading to the deviation from these well-orchestrated processes could result in life-long impacts on the health and development of our brain. Therefore, it is not surprising that extensive efforts have been devoted to identifying critical factors that could influence maturation processes of brain functions, including, but not limited to, environmental factors [14], parent- child interaction [15], and nutrition [16, 17]. In the context of this article, nutritional factors and their effects on normal brain functional development will be discussed. It is widely recognized that adequate nutrition is necessary for normal brain development during pregnancy and infancy, and particular nutrients most likely affect distinct aspects of brain development. However, detailed information on the critical dosage windows and time frames for various nutrients need- ed at different stages of brain development remains lacking. Therefore, approaches capable of assessing the outcomes of exposure to different nutrients, including their timing and dose, are greatly needed, enhancing our understanding of complex interactions between nutrition and brain development through- out early childhood. To this end, this article will first provide a brief overview of several key nutrients in early brain development, followed by how noninvasive imaging methods may provide means to characterize nutritional impacts (both in their timing and dose) on early brain developments, and, finally, a brief over- view of the brain-gut axis.
Nutrition in Early Brain Development
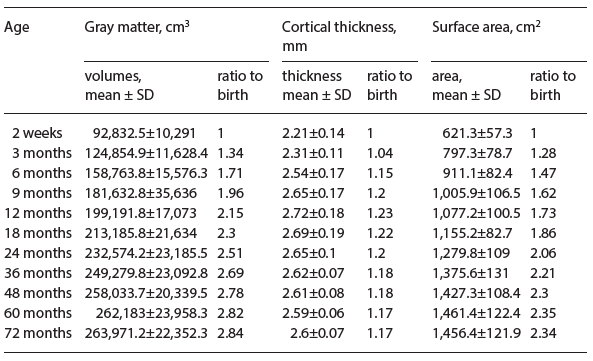
Undoubtedly, nutrition is one of the key factors that could have lifelong impacts on brain cognitive development, particularly during the critical time periods of early brain development. Table 1 summarizes a list of key nutrients and their roles on different aspects of brain development. Speaking to the complexity of this relationship, each nutrient separately affects distinct aspects of brain development at different stages of brain development. For example, long-chain poly- unsaturated fatty acids (LCPUFAs) are essential for both neurogenesis as well as synaptogenesis, affecting both pre- and postnatal brain development. Sphingomyelin is crucial for white-matter (WM) myelination, which undergoes rapid developmental processes from late third trimester throughout the first years of life. It should also be recognized that different nutrients could contribute to similar aspects of brain development, functioning in a complementary or synergistic manner. For example, iron, choline, as well as sphingomyelin have been implicated to play key roles in WM myelination. Given these complex interactions between nutrients and the variable paces of brain structural and functional development, identifying a direct causal relation between nutrients and early brain development has been extremely challenging. Nevertheless, general consensus of potential benefits on early brain development of several essential nutrients has been demonstrated, and a brief review of these key nutrients is provided below.
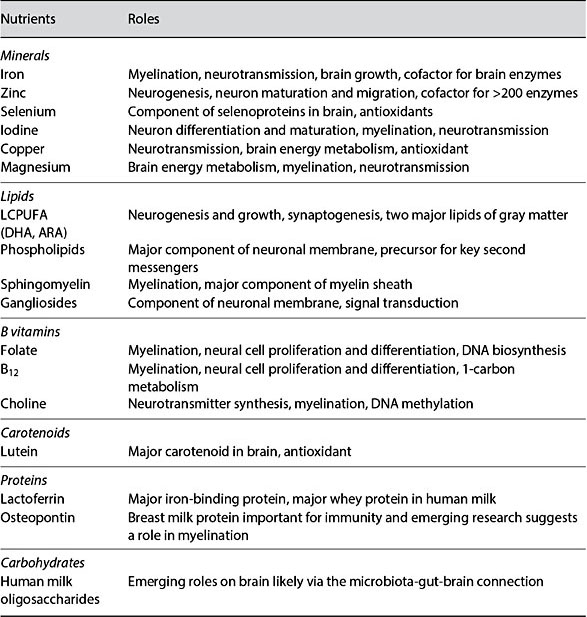
Table 1. Key nutrients and their roles in early brain development
Iron
Iron, as an essential structural component of the hemoglobin molecule, plays a critical role in transporting oxygen throughout the body. The role of iron as a vital nutrient in brain development is highlighted by its sensitive dose-response window; both iron excess and deficiency may induce abnormal brain development. Iron deficiency alters myelination (WM), monoamine neurotransmitter synthesis (striatal-frontal), and neuronal and glial energy metabolism (hippo- campal-frontal) [17]. These brain processes are correlated with developmental behaviors, such as speed of processing (myelination), changes in motor and affect (monoamines), and recognition memory (hippocampus). Infant iron deficiency anemia is a risk factor for both short- and long-term cognitive impairment. These effects may persist even if treatment is provided during infancy, highlighting the gravity of the perinatal role of iron. In multiple longitudinal studies, children with indication of chronic severe iron deficiency in infancy were found to have poorer outcomes than those without. Lack of exposure to iron perinatally resulted in long-term IQ effects, lower motor scores, more grade repetition, anxiety or depression, and social problems [18]. These outcomes persisted despite the introduction of iron therapy in response to anemia in infancy [18]. The mechanism of the perinatal role of iron has been demonstrated robustly in rodent models. Gestational and neonatal iron deficiency resulted in deficits in hippocampal dendritic branching, which persisted in adulthood despite re- storing iron levels to baseline [19]. In addition, even minimal iron deficiency during prenatal and early postnatal development decreases myelin synthesis and alters myelin composition, which is also not corrected with iron therapy [16].
Choline
Choline is a micronutrient highly interrelated with folic acid, vitamin B12, and methionine that is integral in spinal cord and brain development during the perinatal period. Specifically, choline is the precursor of the (1) neurotransmitter acetylcholine, (2) structural phospholipids phosphatidylcholine and sphingomyelin which act as precursors for intracellular messengers, and (3) two signaling lipids, sphingosylphosphocholine and platelet-activating factor. The fetus receives choline across the placenta prenatally and continues to receive high amounts of choline compounds via breast milk postnatally. Rodent studies demonstrate that choline supplementation in utero during the critical window sup- ports hippocampal development (embryonic days 11–18), increases cell proliferation and decreases apoptosis in hippocampal progenitor cells. Rodent studies also show that exposure to extra choline in utero and perinatally leads to hippocampal structure changes that are protective against the cognitive and behavioral effects of prenatal stress and alcohol exposure [20], and even result in life- long improvements in long-term potentiation and visuospatial auditory memory [21]. In a retrospective study, women in the lowest quartile of daily choline intake had a 4-fold greater risk of having a baby with a neural tube defect than the women in the highest quartile for intake [22]. While the mechanism through which choline contributes to permanent changes has not been fully elucidated, the likely mechanisms for the effects of choline come from the cascade effects of its role in DNA methylation, altered gene expression, and the downstream-as- sociated change in stem cell proliferation and differentiation.
Long-Chain Polyunsaturated Fatty Acids
The LCPUFAs, such as docosahexaenoic acid (DHA, 22:6n-3) and arachidonic acid (ARA, 20:4n-6), are essential for brain development during both the prenatal time period as well as the first 2 years of life [23, 24]. In particular, DHA is highly concentrated in the membrane lipids of the WM and gray matter (GM) of the brain and has been implicated as highly essential for WM myelination. In addition, since DHA is abundant in breast milk, it is not surprising that extensive studies have reported cognitive benefits of DHA, including improved work- ing memory [23] and problem-solving skills [24] in infants receiving DHA-supplemented formula. In particular, numerous studies have evaluated the potential benefits of DHA on visual acuity. The DIAMOND (DHA Intake and Measurement of Neural Development) study, a double-masked randomized controlled clinical trial using a dose escalation design, evaluated the potential beneficial effects of DHA on visual acuity [25]. Improved visual acuity was observed only in the group with DHA supplementation of infant formula at 0.32% of total fatty acids. In contrast, Rosenfeld et al. [26] conducted a meta-analysis of 4 clinical trials focusing on DHA; no effects on Bayley Developmental scores at 18 months of age was observed. However, Hoffman et al. [27] suggested that the ratio of DHA and ARA supplement in formula may be of critical importance in determining the potential outcome of cognitive function. Liao et al. [28] utilized event-related potential (ERP) evaluating cognitive improvements in infants receiving control versus DHA-enriched formula at birth and cognitive assessments at 5.5 years of age. Although behavioral assessments (reaction time and accuracy) reveal no statistical differences among groups, ERP shows that the children receiving enriched formula at birth exhibited a greater P2 amplitude, suggesting better visual functioning when compared to the control group. They subsequently concluded that the limited sensitivity of behavioral assessments may contribute to the observed discrepancies between behavioral assessments and ERP and called for more sensitive approaches. To this end, imaging methods, such as MR (to be discussed below), may provide an alternative and sensitive means to assess brain functional development in the first years of life.
Noninvasive Imaging Approaches
While behavioral and cognitive assessments have been widely employed as the outcome measures for most studies focusing on discerning potential beneficial effects of nutrients on brain functional development, they suffer from several inherent limitations. First, a large sample size is typically needed given the anticipated large interrater and intersubject variability. Second, the behavioral repertoire of young infants is relatively limited, making it difficult to objectively assess higher-order brain functional development. Finally, these approaches do not provide a direct assessment of the neural substrates underlying brain functional maturation. Therefore, a sensitive and objective means to assess brain functional development is greatly needed to be able to rigorously, objectively, and quantitatively assess early brain development.
Magnetic resonance imaging (MRI) is a noninvasive imaging modality that is highly versatile and capable of providing detailed anatomical information with exquisite soft-tissue contrast. MR has no radiation and no known adverse effects, enabling longitudinal and noninvasive characterization of normal brain development. However, MR is highly sensitive to motion artifacts; slight motion could lead to poor image quality. While adult subjects can tolerate the immobility required for MRI, this requirement is clearly difficult for children, particularly for toddlers, to comply. Therefore, while MR is an ideal tool to reveal the underlying neural substrates of early brain development, it is only recently that MRI of typically developing children without sedation has become a reality. With dedicated staff and infrastructures, our team has developed strategies that enable imaging of typically developing children from birth to teens without sedation [29]. In particular, 2 main categories of MRI approaches have been employed to characterize structural and functional development during the first years of life. An overview of these imaging approaches, results on early brain structural and functional maturation during the first years of life, and how these approaches may aid to nutritional research focusing on early brain development will be provided below.
Structural Development during the First Years of Life
The total brain volume increases 101% in the first year, followed by a 15% in- crease in the second year [3]. The increase in GM volume (149%) accounts for the majority of the observed increased total body volume with only about 11% increases in WM during the first year [3]. Furthermore, ample evidence demonstrates links between MR morphological measures and functional maturation. Reduced cortical GM density during adolescence and early adulthood [30] is temporally correlated with postmortem findings of increased synaptic pruning. Significant correlations between GM volume in the frontal lobe and children’s performance on a verbal learning task have also been reported [31]. Similar observations with cortical thickness (CT) have also been made where cortical thinning occurs with age as part of normal brain maturation [32]. Thinning of frontal and parietal cortices is associated with improvements in performance on language tasks [33]. While these previous results underscore the importance of structural features of early brain development, detailed characterization of brain structural growth during the first years of life has been a daunting task, particu- larly using a longitudinal protocol. Figure 1 shows T1w (upper row) and T2w (lower row) images of a subject imaged starting from 2 weeks to 6 years of age, demonstrating the remarkable growth of our brain during the first 6 years of life. Table 2 provides quantitative measures of cortical GM volume, CT, and cortica surface area (SA), where SA × CT = GM volume, from a cohort of typically developing children (2 weeks to 6 years of age) undergoing a longitudinal MRI study. Although both GM volume and CT have been widely used to reveal brain structural growth, relatively few results on SA are available. Therefore, the implications of brain SA expansion in relation to cognitive development are yet to be rigorously determined. In particular, it has been demonstrated that brain atrophy typically observed in elderly and demented patients is largely attributed by CT thinning with little change in brain SA [34]. In contrast, computational models implicate that brain SA expansion is more efficient to facilitate brain connectivity than increasing CT [35]. In the context of early brain development where synaptogenesis is one of the main developmental processes, one would hypothesize that not only marked brain SA expansion is present, but also its increase should outpace cortical thickening. Indeed, carefully examining the results shown in Table 2, several important features emerge. First, the total cortical GM volume has almost doubled by the 9th month, followed by a much slower growth pace after the first year of life. By 5 years of age, only a negligible increase in GM volume is observed. Second, CT increases from 2.21 ± 0.14 mm at birth to 2.72 ± 0.18 mm at 1 year, suggesting cortical thickening during the first year of life. However, CT is reduced (cortical thinning) starting from the 18th month of life till about 3 years of age (2.62 ± 0.07 mm) and finally becomes more stable after 3 years of age. Third, although cortical thickening is observed during the first year of life, the increase in CT is relatively small (23%) compared to the increase in cortical GM volume (115%) at the same time period. In other words, the in- creased cortical GM during the first year of life should be largely attributed by brain SA expansion. Indeed, brain SA exhibits a similar growth trajectory as that of cortical GM volume during the first 6 years of life; a marked increase during the first several years of life, followed by a slower brain SA expansion. Together, our results suggest that brain SA expansion is the dominating factor contributing to an increased cortical GM and underscore the potential biological significance of SA expansion during early brain development. Nevertheless, more studies will be needed to determine the potential cognitive implications of SA expansion.
Although the above discussion on brain structural development only focused on 3 structural parameters, it is worth pointing out that additional anatomical features, including but not limited to cortical folding [36], gyrification [37], and sulcal depth [38], can also be obtained using MRI (Fig. 2), demonstrating the capability of MRI in characterizing early brain development.
Myelination is a critical maturation step to ensure rapid and efficient information communication through WM. Successful connection among brain cortical regions likely plays a critical role for the maturation of brain functions. Using diffusion tensor imaging (DTI), an MRI approach, numerous reports have revealed temporal processes of WM myelination [5, 39, 40]. During the early postnatal period, WM first exhibits significantly lower fractional anisotropy (FA), a quantitative measure of directional water mobility, followed by an increased FA with age [5, 39]. The central WM consistently exhibits a higher FA than the peripheral WM in neonates [39]. Huang et al. [41] reported that limbic fibers developed first while the association fibers developed last and commissural and projection fiber tracts are forming from anterior to posterior regions of the brain. Significant associations were also found for WM tracts that connect brain regions known to support working memory in older children and adults (genu, anterior cingulum, and arcuate fasciculus). Better working memory was associated with higher FA and lower radial diffusivity values in these WM tracts [13].
Despite its wide applicability to characterize WM maturation during early brain development, the spatial resolution of DTI is generally lower (1.5 mm3) than that of anatomical images (0.8 mm3). As a result, its ability to characterize myelin content in cortical GM is substantially limited. Yet, quantitative measures of cortical myelin content have been implicated to be associated with brain cognition; a higher cortical myelin content represents a matured cortex and vice versa. To mitigate this difficulty, Glasser and Van Essen have recently proposed a new approach capable of revealing regional cortical myelin content [42]. Although more studies are needed to identify the links between cortical myelin content and cognition, as shown in Table 1, several nutrients (iron, DHA, choline, and sphingomyelin, for example) have been implicated to be of importance for myelination processes. Therefore, in addition to DTI, measures of myelin contents should be included for the assessments of nutritional impacts on brain cognitive development.
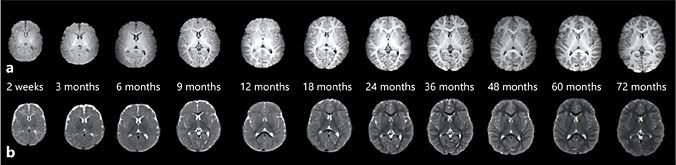
Fig. 1. T1- (a) and T2-weighted (b) MRI scans from a typically developing child longitudinally imaged starting from 2 weeks to 72 months of age.
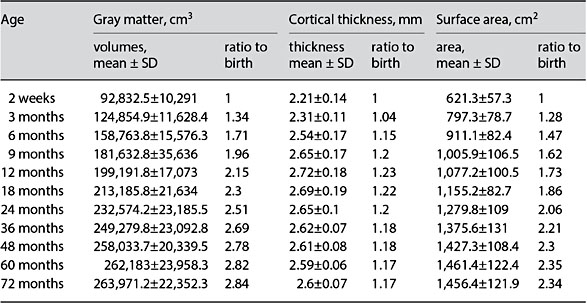
Table 2. Brain structural development during the first 6 years of life
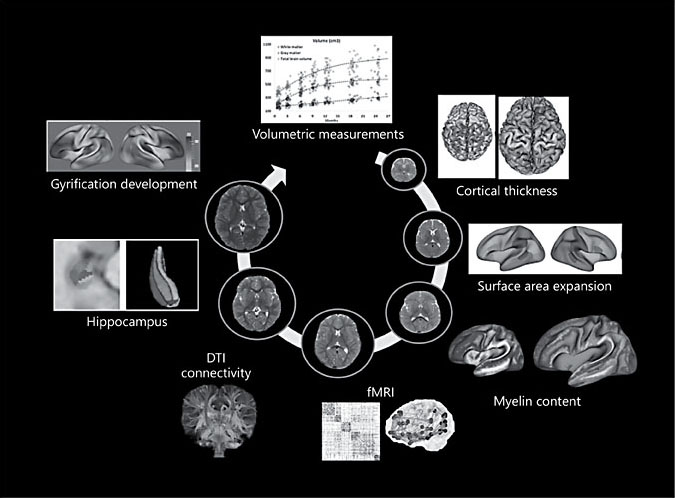
Fig. 2. Examples of essential parameters that can be extracted from MRI approaches to characterize early brain development noninvasively.
Functional Brain Development during the First Years of Life
While it is critically important to determine how different nutrients may impact on structural maturation, equally important is to discern the interplay between cognitive maturation and nutrients during early infancy. Although a number of behavioral and cognitive batteries are currently available to assess brain functional development, as outlined above, these batteries may not be applicable during early infancy. Alternatively, functional MRI (fMRI) or, more specifically, resting state fMRI (rsfMRI) is a perfect tool to characterize brain functional maturation processes in children. In particular, unlike conventional fMRI where a subject’s cooperation to perform predefined cognitive tasks is needed, no specific requirements other than to keep still are needed for rsfMRI. Using rsfMRI, various brain functional networks, including both basic (motor, sensory, visual, and auditory) and higher-order (default mode, attention, executive control, and salience) networks, have been consistently observed in adults. More importantly, other and our teams have employed rsfMRI to examine the development of resting state connectivity in early infancy [9, 43–45]. In a whole-brain topological analysis of 147 healthy subjects from 3 weeks to 2 years of age, we observed small-world topology immediately after birth, with continued maturation during the first 2 years of life [44]. The brain functional topology appears to be more regionally based, without evidence of substantial, long-distance connections be- fore 1 year of age; connectivity becomes more evenly distributed, with increased long-distance connections in year 2 [44]. Furthermore, we reported [46] temporal maturation of major brain functional networks observed in adults during the first year of life, including the sensorimotor (SM), auditory (AN), visual (V1, V2, V3), default mode (DMN), salience (SA), and frontoparietal frontal control (FPC) networks. Based on the growth rates, these networks were classified into 4 different groups. Group 1 includes the AN and SM networks, which are the slowest-growing networks, while group 2 includes V1 and V2, representing the fastest-growing networks. Group 3 includes V3 and DMN, and group 4 includes SA and FPC, reflecting networks with moderate growth rates. Although groups 1 and 4 include networks with slower growth rates, they are distinctly different in functions. It is most likely that group 1 represents the almost matured networks while group 4 reflects the networks that are yet to be developed.
Given the critical role of language function on the development of multiple higher-order functional domains, insights into language maturation during the first years of life could be of critical importance. Homologous language regions (Broca’s and Wernicke’s areas) between the two hemispheres have been report- ed to be functionally connected (functional symmetry) immediately after birth [47, 48]. However, this pattern shifted toward a strong connection between Broca’s and Wernicke’s areas within the same hemisphere but not across the two hemispheres (functional asymmetry), likely reflecting language lateralization [49]. Using a longitudinal design, we evaluated how functional connectivity in the language areas progresses from a symmetric connection at birth to an asymmetric connection between the two hemispheres during early brain development. Both Broca’s and Wernicke’s areas show an increased symmetrical connection in the first year with a peak at approximately 11.5 months of age, fol- lowed by a decrease in the second year. Furthermore, there was a significant correlation between individual’s time to peak symmetry between these two regions (p = 0.011). These findings reveal the complexity and nonlinearity of brain functional developments. More importantly, our results suggest that language lateralization, a critical maturation milestone of language functional development, may be present by the end of the first year of life.
While the aforementioned studies have largely focused on characterizing early brain functional maturation processes without considering factors such as nutrients, these studies demonstrate that rsfMRI can serve as an indispensable tool evaluating how different nutrients may alter functional maturation trajectories during early brain development. Specifically, by collecting breast milk samples, dietary intake, and information on feeding practices concurrently with MR images and detailed behavioral/cognitive assessments, the interaction of these important parameters can potentially be discerned.
Gut-Brain Axis
Recent discovery of bidirectional biochemical signaling between the gastrointestinal tract (including gut flora) and the central nervous system has attracted great interdisciplinary efforts to uncover the gut-brain axis (GBA). While re- search has primarily been conducted in animals thus far, insights have revealed a highly complex communication system that not only maintains gastrointestinal homeostasis [50] but also links peripheral intestine functions to affect motivation and higher cognitive functions [51, 52]. In the absence of microbiota, mice have either an elevated or suppressed stress response level, correlated with abnormal corticosterone concentrations, depending on the wild-type microbiome of the mouse strain [53, 54]. This defect can be returned to baseline with colonization with a specific bacterial species (Bifidobacterium infantis) but not with monocolonization with Escherichia coli, demonstrating that signals from specific bacteria critically impact GBA [54]. In human studies, a 4-week probiotic consumption intervention decreased emotional stimulation in an emotional faces fMRI task, specifically affecting the primary interoceptive and somatosensory regions of the brain [55]. Most relevant to understanding the relation- ship between nutrition and brain development is a recent study that investigated the relationship between gut colonization and brain development for children 1 and 2 years of age. Combining microbiome analysis, MR images, and cognitive testing, human microbial composition at year 1 predicts cognitive performance at year 2 [56]. Further work in understanding GBA is critical in finding out how the axis breaks down in disorders that are already linked to abnormal GI functioning, such as autism, depression, and anxiety [52], as well as understanding the mechanisms for the role of nutrition in brain development and cognition.
Conclusions
The ability to rigorously characterize both structural and functional maturation during the first years of life using MRI is likely to have profound implications on how factors such as nutrition could enhance healthy growth of our brain. The Baby Connectome Project (BCP) – Enriched study, a study specifically designed to discover the complex interaction of nutrition, GBA and early brain maturation, should offer invaluable insights into the interaction of these essential physiological parameters. This knowledge will potentially guide our ability to supplement critical nutrients in early childhood, optimizing both timing and dosage, to promote the healthy growth of our brain during the first years of life.
References
- 1 Casey BJ, Tottenham N, Liston C, Durston S: Imaging the developing brain: what have we learned about cognitive development? Trends Cogn Sci 2005;9:104–110.
- 2 Sowell ER, Trauner DA, Gamst A, Jernigan TL: Development of cortical and subcortical brain structures in childhood and adolescence: a structural MRI study. Dev Med Child Neurol 2002;44:4–16.
- 3 Knickmeyer RC, Gouttard S, Kang C, et al: A structural MRI study of human brain development from birth to 2 years. J Neurosci 2008;28:12176–12182.
- 4 Gilmore JH, Shi F, Woolson SL, et al: Longitudinal development of cortical and subcortical gray matter from birth to 2 years. Cereb Cortex 2012;22:2478–2485.
- 5 Gao W, Lin W, Chen Y, et al: Temporal and spatial development of axonal maturation and myelination of white matter in the developing brain. AJNR Am J Neuroradiol 2009; 30:290–296.
- 6 Kolb B, Gibb R: Brain plasticity and behaviour in the developing brain, J Can Acad Child Adolesc Psychiatry 2011;20:265–276.
- 7 Han J, Kim HJ, Schafer ST, et al: Functional implications of miR-19 in the migration of newborn neurons in the adult brain. Neuron 2016;91:79–89.
- 8 Thompson RA, Nelson CA: Developmental science and the media. Early brain development. Am Psychol 2001;56:5–15.
- 9 Gao W, Zhu H, Giovanello KS, et al: Evidence on the emergence of the brain’s default network from 2-week-old to 2-year-old healthy pediatric subjects. Proc Natl Acad Sci USA 2009;106:6790–6795.
- 10 Gao W, Lin W, Grewen K, Gilmore JH: Functional connectivity of the infant human brain: plastic and modifiable. Neuroscientist 2016,DOI: 10.1177/1073858416635986.
- 11 Elison JT, Wolff JJ, Heimer DC, et al: Frontolimbic neural circuitry at 6 months predicts individual differences in joint attention at 9 months. Dev Sci 2013;16:186–197.
- 12 Kristen S, Sodian B, Thoermer C, Perst H: Infants’ joint attention skills predict toddlers’ emerging mental state language. Dev Psychol 2011;47:1207.
- 13 Short SJ, Elison JT, Goldman BD, et al: Associations between white matter microstructure and infants’ working memory. NeuroImage 2013;64:156–166.
- 14 Calderon-Garciduenas L, Mora-Tiscareno A, Melo-Sanchez G, et al: A critical proton MR spectroscopy marker of Alzheimer’s disease early neurodegenerative change: low hippocampal NAA/Cr ratio impacts APOE ε4 Mexico City children and their parents. J Al- zheimersDis 2015;48:1065–1075.
- 15 Sethna V, Pote I, Wang S, et al: Mother-infant interactions and regional brain volumes in infancy: an MRI study. Brain Struct Funct 2017;222:2379–2388.
- 16 Prado EL, Dewey KG: Nutrition and brain development in early life. Nutr Rev 2014;72: 267–284.
- 17 Georgieff MK: Nutrition and the developing brain: nutrient priorities and measurement. Am J Clin Nutr 2007;85:614S–620S.
- 18 Walker SP, Wachs TD, Gardner JM, et al: International child development steering, child development: risk factors for adverse outcomes in developing countries. Lancet 2007;369:145–157.
- 19 Jorgenson LA, Wobken JD, Georgieff MK: Perinatal iron deficiency alters apical dendritic growth in hippocampal CA1 pyramidal neurons. Dev Neurosci 2003;25:412– 420.
- 20 Thomas JD, La Fiette MH, Quinn VR, Riley EP: Neonatal choline supplementation ameliorates the effects of prenatal alcohol expo- sure on a discrimination learning task in rats. Neurotoxicol Teratol 2000;22:703–711.
- 21 Montoya DA, White AM, Williams CL, et al: Prenatal choline exposure alters hippocampal responsiveness to cholinergic stimulation in adulthood. Brain Res Dev Brain Res 2000; 123:25–32.
- 22 Shaw GM, Carmichael SL, Yang W, et al: Periconceptional dietary intake of choline and betaine and neural tube defects in off- spring. Am J Epidemiol 2004;160:102–109.
- 23 Zhang J, Hebert JR, Muldoon MF: Dietary fat intake is associated with psychosocial and cognitive functioning of school-aged children in the United States. J Nutr 2005;135:1967– 1973.
- 24 Willatts P, Forsyth JS, DiModugno MK, et al: Effect of long-chain polyunsaturated fatty acids in infant formula on problem solving at 10 months of age. Lancet 1998;352:688–691.
- 25 Birch EE, Carlson SE, Hoffman DR, et al: The DIAMOND (DHA Intake and Measurement of Neural Development) Study: a double- masked, randomized controlled clinical trial of the maturation of infant visual acuity as a function of the dietary level of docosahexaenoic acid. Am J Clin Nutr 2010; 91:848–859.
- 26 Rosenfeld E, Beyerlein A, Hadders-Algra M, et al: IPD meta-analysis shows no effect of LC-PUFA supplementation on infant growth at 18 months. Acta Paediatr 2009;98:91–97.
- 27 Hoffman DR, Boettcher JA, Diersen-Schade DA: Toward optimizing vision and cognition in term infants by dietary docosahexaenoic and arachidonic acid supplementation: a review of randomized controlled trials. Prostaglandins Leukot Essent Fatty Acids 2009;81:151–158.
- 28 Liao K, McCandliss BD, Carlson SE, et al: Event-related potential differences in children supplemented with long-chain polyunsaturated fatty acids during infancy. Dev Sci 2017;20:e12455 .
- 29 Lin W, Meng Y, Li Get al: Developmental trajectories of cortical thickness and myelin contents from birth to 6 years old (poster). 22nd Annual Meeting of the Organization for Human Brain Mapping, Vancouver, 2017.
- 30 Sowell ER, Peterson BS, Thompson PM, et al: Mapping cortical change across the human life span. Nat Neurosci 2003;6:309–315.
- 31 Sowell ER, Delis D, Stiles J, Jernigan TL: Im- proved memory functioning and frontal lobe maturation between childhood and adolescence: a structural MRI study. J Int Neuropsychol Soc 2001;7:312–322.
- 32 Giedd JN, Blumenthal J, Jeffries NO, et al: Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci 1999;2:861–863.
- 33 Sowell ER, Thompson PM, Leonard CM, et al: Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci 2004;24:8223–8231.
- 34 Storsve AB, Fjell AM, Tamnes CK, et al: Differential longitudinal changes in cortical thickness, surface area and volume across the adult life span: regions of accelerating and decelerating change. J Neurosci 2014;34: 8488–8498.
- 35 Murre JM, Sturdy DP: The connectivity of the brain: multi-level quantitative analysis. BiolCybern 1995;73:529–545.
- 36 Li G, Wang L, Shi F, et al: Construction of 4D high-definition cortical surface atlases of infants: methods and applications. Med Image Anal 2015;25:22–36.
- 37 Li G, Wang L, Shi F, et al: Mapping longitudinal development of local cortical gyrifica- tion in infants from birth to 2 years of age. J Neurosci 2014;34:4228–4238.
- 38 Meng Y, Li G, Lin W, et al: Spatial distribution and longitudinal development of deep cortical sulcal landmarks in infants. Neuro- Image 2014;100:206–218.
- 39 Zhai G, Lin W, Wilber KP, et al: Comparisons of regional white matter diffusion in healthy neonates and adults performed with a 3.0-T head-only MR imaging unit. Radiology 2003;229:673–681.
- 40 Chen Y, Zhu H, An H, et al: More insights into early brain development through statistical analyses of eigen-structural elements of diffusion tensor imaging using multivariate adaptive regression splines. Brain Struct Funct 2014;219:551–569.
- 41 Huang H, Zhang J, Wakana S, et al: White and gray matter development in human fetal, newborn and pediatric brains. NeuroImage 2006;33:27–38.
- 42 Glasser MF, Van Essen DC: Mapping human cortical areas in vivo based on myelin content as revealed by T1- and T2-weighted MRI. J Neurosci 2011;31:11597–11616.
- 43 Lin W, Zhu Q, Gao W, et al: Functional connectivity MR imaging reveals cortical functional connectivity in the developing brain. AJNR Am J Neuroradiol 2008;29:1883–1889.
- 44 Gao W, Gilmore JH, Giovanello KS, et al: Temporal and spatial evolution of brain net- work topology during the first two years of life. PLoS One 2011;6:e25278.
- 45 Gao W, Gilmore JH, Shen D, et al: The synchronization within and interaction between the default and dorsal attention networks in early infancy. Cereb Cortex 2013;23:594–603.
- 46 Gao W, Alcauter S, Elton A, et al: Functional network development during the first year: relative sequence and socioeconomic correlations. Cereb Cortex 2015;25:2919–2928.
- 47 Perani D, Saccuman MC, Scifo P, et al: Neural language networks at birth. Proc Natl Acad Sci USA 2011;108:16056–16061.
- 48 Thomason ME, Dassanayake MT, Shen S, et al: Cross-hemispheric functional connectivity in the human fetal brain. Sci Transl Med 2013;5:173ra124.
- 49 Lohmann G, Hoehl S, Brauer J, et al: Setting the frame: the human brain activates a basic low-frequency network for language processing. Cereb Cortex 2010;20:1286–1292.
- 50 Carabotti M, Scirocco A, Maselli MA, Severi C: The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol 2015;28:203–209.
- 51 Foster JA, McVey Neufeld KA: Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci 2013;36: 305–312.
- 52 Song Y, Liu C, Finegold SM: Real-time PCR quantitation of clostridia in feces of autistic children. Appl Environ Microbiol 2004;70: 6459–6465.
- 53 Sampson TR, Mazmanian SK: Control of brain development, function, and behavior by the microbiome. Cell Host Microbe 2015; 17:565–576.
- 54 Sudo N, Chida Y, Aiba Y, et al: Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol 2004;558:263–275.
- 55 Tillisch K, Labus J, Kilpatrick L, et al: Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology2013;44:1394–1401, 1401.e1–4.
- 56 Carlson AL, Xia K, Azcarate-Peril MA, et al: Infant gut microbiome associated with cognitive development. Biol Psychiatry 2018;83: 148–159.