Do Probiotics Really Need to Be “Alive”?
Do Probiotics Really Need to Be “Alive”?
Yvan Vandenplas
Key Messages
- The potential beneficial health effects of “not live” microorganisms deserve to be better studied as their production and preservation are much easier than for living organisms
- Some data suggest that particles of microorganisms and/or their metabolites may be sufficient to induce immunological effects
- More evidence is needed regarding the potential benefits of non-viable microorganisms
What does “probiotic” mean? Some researchers give the word a full Greek etymology, but it appears to be a composite of the Latin preposition pro (“for”) and the Greek adjective ß..t.... (biotikos, “fit for life, lively”), the latter deriving from the noun ß... (bios, “life”). A probiotic is defined as living microorganisms that, when consumed in adequate amounts, has a health benefit for the host. Although the definition of a probiotic requires that the microorganisms ingested are “alive,” the question arises if dead microorganisms cannot also have a health benefit. It is beyond any doubt that the evidence for beneficial health effects of “living” bacteria is much larger and stronger than for “dead” bacteria. A major reason for this difference is due to the simple fact that living bacteria have been studied much more than dead bacteria. What do we know about “not living bacteria”?
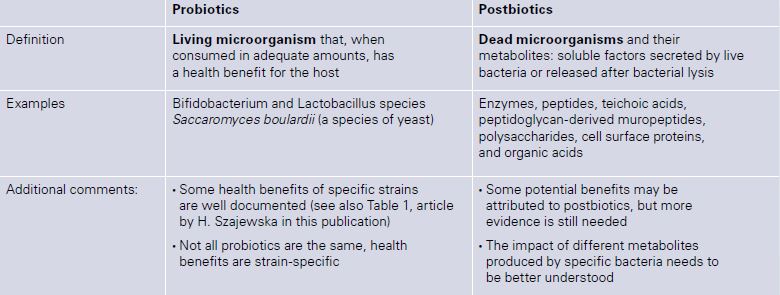
Literature on the efficacy of heat-killed probiotic microorganisms is booming. Lactobacillus LB, for example, are used in the treatment of acute gastroenteritis [1]. Other products have been shown to be effective in the treatment of infantile colic [2]. Spore-based probiotics, or sporebiotics consist of the cell wall of bacillus microorganisms. The lay-publicity and claims for sporebiotics are impressive: “Because of the sporebiotics’ unique abilities, scientists believe they may benefit people with health problems such as autism, neurological disorders, and immune-related diseases. In addition, spore-based probiotics may help fortify the body against environmental aggressors such as electromagnetic fields (EMFs), pesticides, and airborne pollutants.” However, there is hardly any information in the scientific literature. The term postbiotic was introduced to describe a product containing dead microorganisms and their metabolites: soluble factors (products or metabolic by-products), secreted by live bacteria, or released after bacterial lysis, such as enzymes, peptides, teichoic acids, peptidoglycan-derived muropeptides, polysaccharides, cell surface proteins, and organic acids. Some dead probiotics have been shown to modulate the immune system (compounds of the cell wall might boost the immunological system) and to have increased adhesion to intestinal cells which further results in inhibition of pathogens (Table 1).
Table 1: Main characteristics of probiotics and postbiotics
Fermented infant formula has been widely available in many countries for a few decades. It contains bacteria that were “killed” during the production/fermentation process, resulting in the presence of dead bacteria and metabolites in the final product [3]. Five randomized controlled trials involving 1,326 infants compared the use of fermented formula with non-fermented routine formula and showed that weight and length gain during the study period were similar. Fermented infant formula also has the potential to reduce some digestive symptoms. Current evidence does not support the use of fermented formula for preventing cow’s milk allergy [3].
In conclusion, potential beneficial health effects of dead microorganisms deserve to be better studied as the production and preservation of dead microorganisms are much easier than for living organisms. Although it seems likely that living microorganisms are needed to restore or influence the intestinal mi-crobiome composition, it seems also likely that particles of microorganisms and/or their metabolites may be sufficient to induce immunological effects. However, more evidence is needed regarding the potential benefits of nonviable microorganisms. Legislation and claims are running far behind the imagination of marketeers.
References
- Salazar-Lindo E, Figueroa-Quintanilla D, Caciano MI, Reto-Valiente V, Chauviere G, Colin P; Lacteol Study Group. Effectiveness and safety of Lactobacillus LB in the treatment of mild acute diarrhea in children. J Pediatr Gastroenterol Nutr. 2007 May;44(5):571–6.
- Vandenplas Y, Bacarea A, Marusteri M, Bacarea V, Constantin M, Manolache M. Efficacy and safety of APT198K for the treatment of infantile colic: a pilot study. J Comp Eff Res. 2017 Mar;6(2):137–44.
- Szajewska H, Skórka A, Pies´cik-Lech M. Fermented infant formulas without live bacteria: a systematic review. Eur J Pediatr. 2015 Nov;174(11):1413–20.
