Compositional Analysis and Metabolism of Human Milk Oligosaccharides in Infants
Abstract
It is a great success that biotechnological means are available today to produce amounts of single human milk oligosaccharides (HMOs) in a purity which allows performing metabolic and functional studies even in humans. As recent data indicate that there is a link between the Lewis blood group and the secretor status of an individual and certain inflammatory diseases, this review will also focus on the metabolic fate of secretor- and Lewis blood group-specific components. We conclude that there is no simple urinary or fecal excretion pattern of HMOs, although the pattern in urine often reflects the mother’s secretor/nonsecretor status. However, there are deviations for single HMOs which deserve special attention. In feces, the variation in excretion is much higher than in urine, which may be caused by variations in the infant’s intestinal microbiota. A gradual decrease in HMO excretion with time as proposed earlier does not take place as even after 7 months of exclusive breastfeeding often intact HMOs can be detected in feces and urine. In addition, we found that whenever oligosaccharides were detected in feces, LNT, the major core structure of HMOs, was present. Hence, our data do not support speculations that LNT is a preferable source for the microbiota.
Introduction
Already around 1900, concomitant with the discovery of lactobacilli and bifidobacteria and their relevance for health and disease, pediatricians realized that the microbial composition of stool samples from breastfed and bottle-fed infants differed. Observations indicated that this difference was particularly linked to the milk carbohydrate fraction. This was the starting point of research on human milk carbohydrates. In the middle of the last century, the first human milk oligosaccharides (HMOs) were identified by Richard Kuhn in Heidelberg (Germany) [1]. At the same time, Paul György’s studies in Heidelberg and later in Philadelphia (USA) focused on the identification of various HMOs as the “bifidus factor” in human milk ( Fig. 1 ) [2]. Meanwhile about 150–200 single HMOs have been characterized.
In recent years, there has been a tremendous increase in our knowledge regarding specific effects of HMOs [3–10]. Even the first human studies on infant formula supplemented with single HMOs have already been published [11, 12]. In order to determine which compound(s) would be most suitable for supplementation, in which concentrations or combinations, and how long they should be given, studies are needed regarding the metabolic fate of HMOs as well as their local and systemic effects.
A straight forward strategy to investigate the metabolism of HMOs is to determine the presence or absence of natural milk oligosaccharides in feces, blood, urine, or tissue. However, some prerequisites hamper this simple approach as, for example, tissue and blood are not available or rather difficult to obtain from human infants. Also the methodological handling of a large number of samples is still a difficult task and requires standardized methods.
In the following, we briefly address the difficulties of compositional analysis before we present a summary of metabolic aspects from various studies which focus on the excretion of oligosaccharides in feces and urine.
Compositional Analysis of Human Milk Oligosaccharides – Need for Standardized Methods
Standardized methods for routine analysis are not available yet, which is one major reason for the discrepancy between reported qualitative and quantitative data. For the identification of HMOs, various methods such as paper chromatography, HPLC coupled with mass spectrometry (MS), HPAEC-PAD (high-performance anion exchange chromatography), HPLC chip MS, capillary gel electrophoresis/laser-induced fluorescence techniques, or liquid chromatography-HPLC-MS have recently been used. All these methods provide detailed in-sights into the oligosaccharide pattern of individual milk samples, frequently paired with further information about the relative amount of single isomers. However, most of these techniques require a sophisticated and time-consuming sample preparation, which is a drawback for the analysis of large sample sets.
With regard to quantitative aspects, data from different laboratories also vary significantly [13–17]. Frequently, various MS methods are applied, and concentrations are given as “relative data”. However, MS can hardly be recommended for quantitative purposes, and interpretation of those data should be done with caution [18]. In addition, there is the need for using laborious derivatization techniques, such as permethylation or labeling with fluorescence reagents. As a consequence of quantitative considerations, it would be necessary to prove the efficiency of derivatization after each single step. Also, it has to be kept in mind that often a time-consuming sample preparation with various centrifugation and/or extraction steps using different cartridges has to be performed before applying the sample to an MS instrument. The efficiency of those requirements may vary, for example, between individuals or laboratories, issues which should certainly be solved as soon as possible.
Metabolism of Human Milk Oligosaccharides
The high structural diversity of HMOs complicates acquiring a sophisticated knowledge on “what structures are important for infants’ health” despite the recent methodological
developments to characterize minute amounts of HMOs and their degradation products in biological samples. Metabolic studies can help to identify if HMOs are absorbed and utilized by the infant’s organism. Moreover, fecal oligosaccharide profiling might give an indication of the intestinal health of infants due to the known relationship be-tween microbiota and the abundance of complex glycans in the infant’s gut. Urinary excretion can support observations from studies addressing systemic effects of oligosaccharides.
Only recently, studies trying to prove a direct link between HMO structures and their prebiotic function in vivo in infants have been published. A recent proof-of-concept study indicated an association between the fecal HMO composition and gut microbiota of 2 breastfed infants over time, though both parameters differed substantially between both infants studied [19]. Another study showed a relationship between the fecal microbiome of exclusively breastfed infants and the HMO composition of the milk that was fed [20].
However, since the variation in both (HMOs and microbiota composition) is vast, unequivocal structure-function studies are inevitable for a better understanding of their association with health and disease. First studies on fecal oligosaccharides and possible metabolites of HMOs in infants have already been reported by Lundblad’s group in the 1970s [21]. Recent studies using new technologies either included only one or a low number of infants, or showed data from premature infants whose intestinal function may be very different from healthy term infants [22, 23] . Current data have so far led to the conclusion that the major portion of HMOs reaches the lower gastrointestinal tract where HMOs might be used as nutritive factors by various microorganisms or influence their activities. The remainders are excreted intact or in metabolized form in feces or urine, indicating that there is no uniform metabolic process for all HMOs [5, 24, 25] .
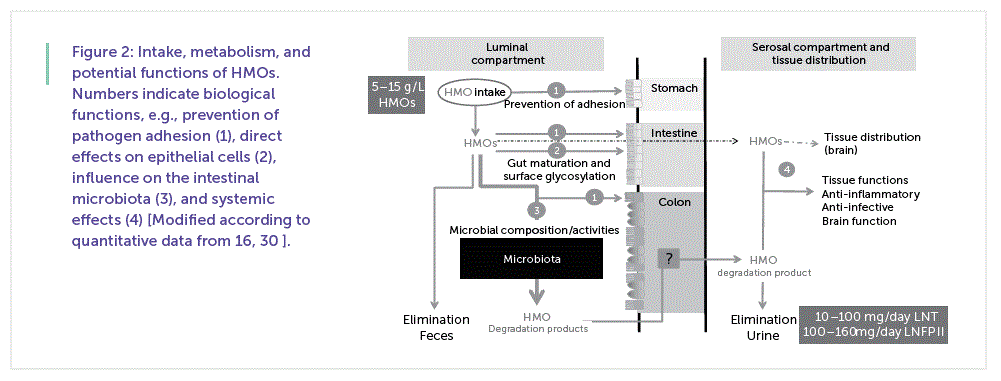
Figure 2 shows a summary of the intake, metabolism, and examples for potential functions of HMOs currently being discussed.
Content of Human Milk Oligosaccharides in Term and Preterm Milk
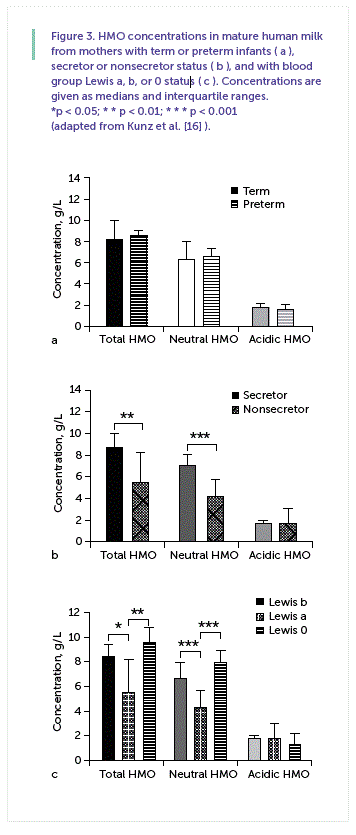
To further improve infant formulas in order to gain more benefit from HMOs, a question often raised is how much of an individual component should be supplemented. In this context, some studies indicated that one has to differentiate between term and preterm milk, as the latter is considered to have either a higher content or a different pattern than term milk [15, 23]. As we recently discussed, we found no difference in the total amount of HMOs between term and preterm milk, neither in colostrum nor in transitory or mature milk, which is exemplified for mature milk in Figure 3 a [16] . Often, however, no distinction is made between secretor and nonsecretor milk among the pool of samples analyzed, which will be further discussed in the following.
Intake of Human Milk Oligosaccharides – Differentiation between Lewis Blood Group and Secretor/Nonsecretor Milk Status
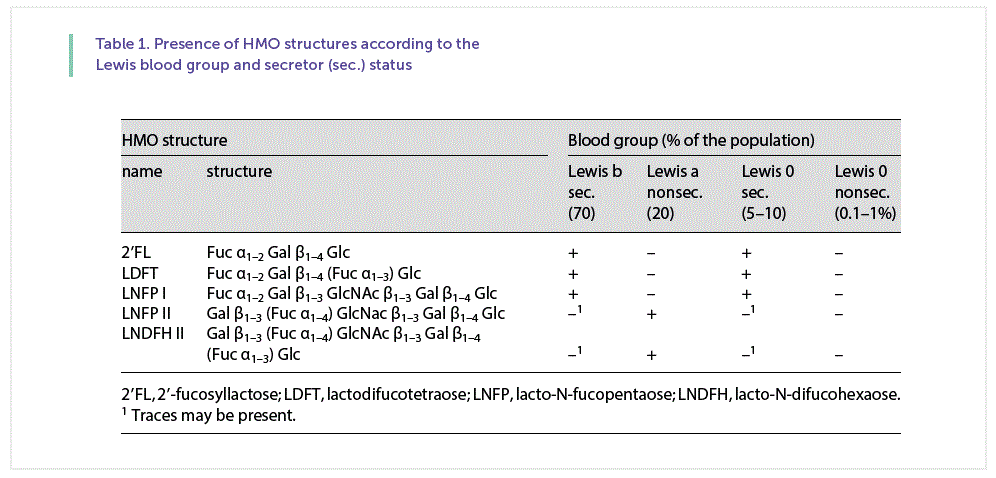
It has been established for a long time that the HMO pattern depends on the genetic background of the mother, i.e., on the Lewis blood group and the secretor status ( Table 1 ) [26, 27]. This aspect is particularly important as feeding infants with a different HMO pattern might reduce or even increase the risk for certain diseases. Grouping the same sample set of term and preterm milk ( Figure 3 a) according to the secretor status and the Lewis blood group of the mother, it is obvious that there are large differences between milk samples depending on the secretor and Lewis blood group specific pattern ( Fig. 3 b, c). Differences between individual HMOs in both groups and in Lewis a, Lewis b and Lewis-negative milk samples are further discussed by Kunz et al. [16] .
Comparison of Human Milk Oligosaccharides in Milk, Urine,
and Feces in Mother-Child Dyads
We performed 2 stable isotope studies collecting milk, feces, urine, and breath samples at each suckling over up to 36 h after having applied an oral bolus of 13 C-labeled glucose or galactose given to lactating mothers [28–31]. In the following, we present some of these data together with those from 2 other cohort studies [16, 32].
Urinary Excretion Pattern
Our data so far support the general conclusion that the overall pattern of neutral oligosaccharides in the urine from breastfed infants reflects that of their mothers’ milk
suggesting a strong association with the mothers’ Lewis blood group and secretor phenotype. The major difference between the milk from mothers with blood group Lewis a, Lewis b, and Lewis 0 is a rather complex pattern in “Lewis b milk” compared to “Lewis a milk,” and “Lewis negative milk” [25, 31, 32, 33]. Comparing HMOs from “Lewis a” or “Lewis b milk” with the urinary oligosaccharide pattern in the corresponding infants, for example, a similar pattern with the same molecular masses was observed [25, 30, 31].
Using MALDI-TOF (matrix assisted laser desorption ionization-time of flight)-MS, differences could be seen in the signal intensities for both, milk and urine, although the masses m/z 730 (LNT, LNnT), 876 (monofucosylated LNT/ LNnT), or 1022 (difucosylated LNT/LNnT), as well as others, are present in all samples. As lacto-N-fucopentaose (LNFP) I is characteristic for “Lewis b milk,” one would expect that m/z 876, a major mass signal, represents this component; however, MS does not directly allow a clear assignment of various isomeric structures. Analyzing the fraction by HPAEC with pulsed amperometric detection enabling a separation of HMO isomers such as LNFP I and II, we were not able to detect LNFP I in any of the investigated urine samples from infants receiving “Lewis b milk” from their mothers [25, 31] .
Fecal Excretion Patterns – Changes with Time
Oligosaccharides known from human milk can be detected in many fecal samples from exclusively breastfed infants [26, 33]. Sometimes the feces of infants displayed a profile distinguishable from milk, where certain HMOs predominated and common, especially larger HMOs, were clearly diminished, and, often, no HMOs were detected, especially after 6–7 months of breastfeeding.
Some infants had reduced abundances of larger structures and a remarkably high proportion of difucosylated LN(n)T (isomers) at m/z 1022, which, in contrast, could not be detected in other infants. The predominance of the signal at m/z 1022 was also reported by Albrecht et al. [34] in 1 infant. In other cases, however, we observed lower fecal HMO excretions in earlier samples (2 months of age) compared to follow-up samples (7 months of age). This observation is contradictory to the reports of Albrecht et al. [34] , who claimed a gradual decrease in HMO abundance with age, hypothesizing an association with gut (microbiota) maturity [22] .
Furthermore, one would expect that secretor-specific HMOs would only be present in feces or urine of infants fed milk from “secretor mothers” or “Lewis b mothers.” However, we identified secretor-specific oligosaccharides 2’FL, DFLT, and LNDFH I not only in urine and feces from infants fed “secretor milk,” but often also in those fed “nonsecretor milk” [25]. This finding is in line with reports by Lundblad’s group [21] for feces and the study by De Leoz et al. [35] for urine.
In addition and contradictory to other publications, we detected LNT and LNnT, 2 structures commonly present in human milk, in many infants’ urine, and, if HMOs were present, in all fecal samples. In other reports on fecal samples, however, LNT was absent in samples from breastfed infants at different time points [22, 35].
Perspectives
It can be concluded that there is no simple urinary or fecal excretion pattern of HMOs, although the pattern in urine often reflects the mother’s secretor/non-secretor status. However, there are obviously deviations for some single HMOs which deserve special attention. In feces, the variation in excretion is much higher than in urine, which may be caused by variations in the microbial composition within the gastrointestinal tract. A simple gradual decrease in HMO excretion with time as proposed earlier does not take place, as even after 7 months of exclusive breastfeeding intact HMOs can be detected, although in the majority of infants no oligosaccharides are present. In addition, we found that whenever oligosaccharides were detected in feces, LNT, the major core structure of HMOs, was present. Hence, our data do not support previous speculations that LNT is a preferable source for microbiota.
In general, large amounts of HMOs, i.e., several grams per day, reach the gastrointestinal tract of a human milk-fed infant. About 1–5% of HMOs seem to be excreted via infants’ urine [19, 28, 29, 31, 36]. Determining the amount of individual HMOs in urine, about 50–160 mg LNT or LNFP II, for example, can be found, a clear indication that HMOs were absorbed [30]. Hence, several hundred milligrams of HMOs per day circulate in the infant’s blood. Therefore, it can be expected that not only local effects of HMOs within the gastrointestinal tract but also systemic functions may occur.
Data presented so far raise the question what it means using human milk as the gold standard when it comes to a standard for the composition of HMOs. There are large differences in the total amount and in the pattern of individual HMOs based on the genetic background of the mother. Is the quality of human milk minor when specific HMOs are missing as it is the case in nonsecretors, which comprise about 20% of the population? Do nonsecretor infants deserve special attention assuming that they may have a higher risk for developing certain diseases? Therefore, it may not only be necessary to identify HMOs with the highest benefit for most infants, but also to determine the (health/disease) risk of infants based on their (im)maturity at birth as well as their own genetic background to improve the benefit of HMO supplementation.
References
1 Kuhn R: Les oligosaccharides du lait. Bull Soc Chim Biol 1958; 40: 297–314.
2 György P: A hitherto unrecognized biochemical difference between human milk and cow’s milk. J Pediatr 1953; 11:98–108.
3 Kunz C: Historical aspects of human milk oligosaccharides. Adv Nutr 2012; 3: 430S–439S.
4 Egge H: The diversity of oligosaccharides in human milk; in Renner B, Sawatzki G (eds): New Perspectives in Infant Nutrition. Stuttgart/New York, Thieme, 1993, pp 12–26.
5 Bode L: Human milk oligosaccharides: every baby needs a sugar mama. Glycobiology 2012; 22: 1147–1162.
6 Asakuma S, Hatakeyama E, Urashima T, et al: Physiology of consumption of human milk oligosaccharides by infant gut-associated bifi- dobacteria. J Biol Chem 2011; 286: 34583–34592.
7 Kunz C, Kuntz S, Rudloff S: Bioactivity of human milk oligosaccharides; in Moreno FJ, Sanz ML (eds): Food Oligosaccharides: Production, Analysis and Bioactivity. Oxford, Wiley-Blackwell, 2014, pp 5–20.
8 Sela DA, Mills DA: Nursing our microbiota: molecular linkages between bifidobacteria and milk oligosaccharides. Trends Microbiol 2010; 18: 298–307.
9 Urashima T, Kitaoka M, Terabayashi T, et al: Milk oligosaccharides; in Gordon NG (ed): Sources, properties and applications. New York, Nova Science Publishers, 2011, pp 1–77.
10 Donovan SM, Wang M, Li M, et al: Host-mi crobe interactions in the neonatal intestine: role of human milk oligosaccharides. Adv Nutr 2012; 3: 450S–455S.
11 Marriage BJ, Buck RH, Goehring KC, et al: Infants fed a lower calorie formula with 2’ FL show growth and 2’ FL uptake like breast- fed infants. J Pediatr Gastroenterol Nutr 2015; 61: 649–658.
12 Steenhout P, Sperisen P, Martin F-P, et al: Term infant formula supplemented with hu-man milk oligosaccharides (2’ fucosyllactose and lacto-N-neotetraose) shifts stool micro-biota and metabolic signature closer to that of breastfed infants (poster). Exp Biol, 2016.
13 Asakuma S, Urashima T, Akahori M, et al: Variation of major neutral oligosaccharides levels in human colostrum. Eur J Clin Nutr 2007; 62: 488–494.
14 Chaturvedi P, Warren CD, Altaye M, et al: Fucosylated human milk oligosaccharides vary between individuals and over the course of lactation. Glycobiology 2001; 11: 365–372.
15 Gabrielli O, Zampini L, Galeazzi T, et al: Pre-term milk oligosaccharides during the first month of lactation. Pediatrics 2011; 128:e1520–e1531.
16 Kunz C, Meyer C, Collado MC, et al: Influence of gestational age, secretor and Lewis blood group status on the oligosaccharide content of human milk. J Pediatr Gastroen-terol Nutr, Epub ahead of print.
17 Nakhla T, Fu D, Zopf D, et al: Neutral oligosaccharide content of preterm human milk. Br J Nutr 1999; 82: 361–367.
18 Duncan MW: Good mass spectrometry and its place in good science. J Mass Spectrom 2012; 47: 795–809.
19 DeLeoz MLA, Kalanetra KM, Bokulich NA, et al: Human milk glycomics and gut micro-bial genomics in infant feces show a correlation between human milk oligosaccharides and gut microbiota: a proof-of-concept study. J Proteome Res 2015; 14: 491–502.
20 Wang M, Li M, Wu S, et al: Fecal microbiota composition of breast-fed infants is correlat-ed with human milk oligosaccharides consumed. J Pediatr Gastroenterol Nutr 2015; 60: 825–833.
21 Sabharwal H, Nilsson D,Grönberg G, et al: Oligosaccharides from feces of preterm infants fed on breast milk. Arch Biochem Bio-phys 1988; 265: 390–406.
22 Albrecht S, Schols HA, Van den Heuvel EGHM, et al: CE-LIF-MS n profiling of oli-gosaccharides in human milk and feces of breast-fed babies. Electrophoresis 2010; 31: 1264–1273.
23 De Leoz MAL, Gaerlan SC, Strum JS, et al: Lacto-N-tetraose, fucosylation, and secretor status are highly variable in human milk oli-gosaccharides from women delivering pre-term. J Proteome Res 2012; 11: 4662–4671.
24 Rudloff S, Kunz C: Milk oligosaccharides and metabolism in infants. Adv Nutr 2012; 3: 398–405.
25 Dotz V, Rudloff S, Meyer C, et al: Metabolic fate of neutral human milk oligosaccharides in exclusively breast-fed infants. Mol Nutr Food
Res 2015; 59: 355–364.
26 Le Pendu J: Histo-blood group antigen and human milk oligosaccharides: genetic poly-morphism and risk of infectious diseases. Adv Exp Med Biol 2004; 554: 135–143.
27 Prieto PA: Profiles of human milk oligosaccharides and production of some human milk oligosaccharides in transgenic animals. Adv Nutr 2012; 3: 456S–464S.
28 Rudloff S, Pohlentz G, Diekmann L, et al: Urinary excretion of lactose and oligosaccha-rides in preterm infants fed human milk or infant formula. Acta Paediatr 1996; 85: 598–603.
29 Rudloff S, Obermeier S, Borsch C, et al: Incorporation of orally applied 13 C-galactose into milk lactose and oligosaccharides. Gly-cobiology 2006; 16: 477–487.
30 Rudloff S, Pohlentz G, Borsch C, et al: Urinary excretion of in vivo 13 C-labelled milk oligosaccharides in breastfed infants. Br J Nutr 2012; 107: 957–963.
31 Dotz V, Rudloff S, Blank D, et al: 13 C-labeled oligosaccharides in breastfed infants’ urine: individual-, structure- and time-dependent differences in the excretion. Glycobiology 2014; 24: 185–194.
32 Dotz V, Adam R, Lochnit G, et al: Neutral oligosaccharides in feces of breastfed and formula-fed infants at different ages. Glycobiology 2016; 26: 1308–1316.
33 Blank D, Gebhardt S, Maass K, et al: High-throughput mass finger printing and Lewis blood group assignment of human milk oli-gosaccharides. Anal Bioanal Chem 2011; 401: 2495–2510.
34 Albrecht S, Schols HA, van den Heuvel EGHM, et al: Occurrence of oligosaccharides in feces of breast-fed babies in their first six months of life and the corresponding breast milk. Carbohydr Res 2011; 346: 2540–2550.
35 De Leoz MLA, Wu S, Strum JS, et al: A quantitative and comprehensive method to analyze human milk oligosaccharide structures in the urine and feces of infants. Anal Bioanal Chem 2013; 405: 4089–4105.
36 Goehring KC, Kennedy AD, Prieto PA: Direct evidence for the presence of human milk oligosaccharides in the circulation of breast-fed infants. PLoS One 2014; 9:e101692.
37 Rose CS, Kuhn R, Zilliken F, Gyorgy P: Bifi dus factor. V. The activity of α- and β-methyl-N-acetyl- D-glucosaminides. Arch Biochem Biophys 1954; 49: 123–129.