Clinical Studies: What is known and what needs further studies
HMO are the third most important component in human milk, more important as solid than protein fraction. And that is a big difference with infant formula and cow‘s milk, in which this part is not present.
The oligosaccharides are very complex,very abundant and the proportion is not similar during the whole period of lactation.
More than 150 different HMOs have been identified. To more simplify the 150 we can according to the different monosaccharides present them in three categories. (Chart 1)
Two of those are more abundant: 2`FL and LNnT. And from in vitro studies we know that there is accumulating evidence suggesting that they may support gastrointestinal and immune functions of breastfed infants. The mechanisms that have been put forward are the effect of the microbiota growth, function and establishment, protection from infection and effects on allergy and immune competence. One of the ways why people think that they might help against infections and bacterial infections is that they have similarities with some receptors of certain gastrointestinal bacteria and they could interact or prevent the adherence of those microbes to the receptors in our gut.
We performed in two centers in Palermo, Italy, and in Hasselt, Belgium, this randomized controlled trial with infant formula supplemented with 2 synthetic human milk oligosaccharides (Alliet et al JPGN 2017; 64: 624-631).
The overall objective of that safety study was the effect of this formula supplemented with these 2 synthetic human milk oligosaccharides on growth, digestive tolerance and morbidity in healthy infants. For the microbiota related objectives we compare microbiota profile and metabolic signature among the infants given the test formula (EF) and the control formula (CF) and there was also a non- randomized exclusively breastfed infants group (BF).
The hypothesis was that microbiota profile and metabolic signature in the infants fed EF (vs. CF) will be closer to that of BF infants. Infants were enrolled before the age of 2 weeks. After informed consent they got a medical examination and anthropometry. Then they were randomized towards the test or the control formula. (Chart 2)
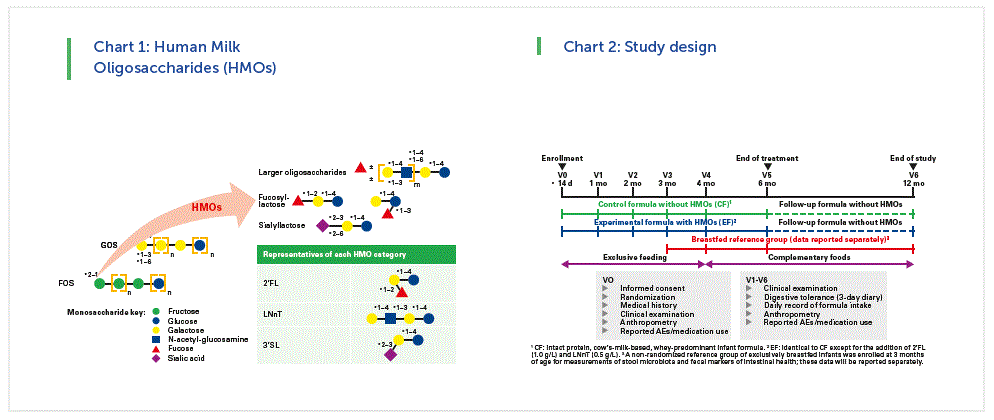
They were seen at 1, 2, 3, 4 and 6 months, which was the end of the treatment. All infants received a standard follow-up formula from 6-12 months and were seen again at 12 months. Complementary food was only started at 4 months of age, digestive tolerance and adverse effect were recorded in a parent’s diary. (Chart 3)

Baseline characteristics
175 infants were included, most of them were followed up at 6 and 12 months. Baseline characteristics were comparable between EF and CF groups. Mean difference in weight gain (EF vs. CF) was -0.30 g/day (95% CI -1.94– 1.34, p=0.72), within the non-inferiority margin. Infants receiving EF (vs. CF) did not differ in weight, length, head circumference, BMI, or corresponding z-scores through 12 months. Digestive Tolerance Measures of digestive tolerance were generally similar between groups:
- no difference in stool consistency, except at 2 mo (softer in EF vs. CF)
- no difference in stool frequency between EF and CF groups
- GI symptoms and behavioral patterns were similar between groups
The bacterial composition
There was a big distinction between EF and CF groups.
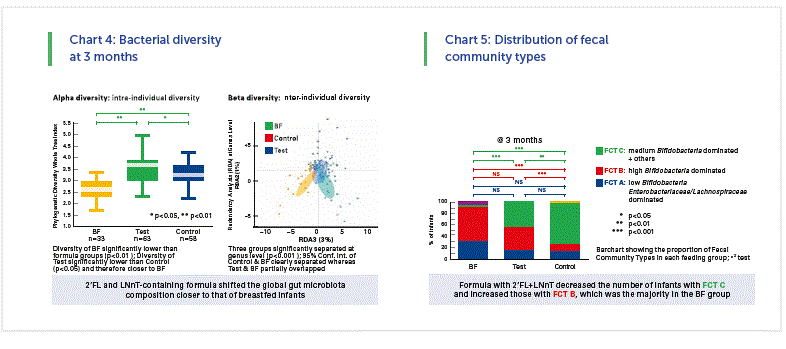
- 2’FL and LNnT-containing formula shifted the global gut microbiota composition closer to that of breastfed infants. (Chart 4)
- Formula with 2’FL and LNnT promoted the colonisation of potentially beneficial Bifidobacterium and reduced taxa with potentially pathogenic members.
- Stool metabolic signature of EF was closer to BF, consistent with the findings in bacterial composition and diversity.
- Altered bacterial composition may result in reduced protein fermentation in EF vs. CF.
Clinical results
The only way we could look at the clinical results in this study was by using the reported adverse events,identified a priori, and medication use.
• Infants receiving EF (vs. CF) were less likely to experience bronchitis through 4 months (OR 0.16, 95% CI 0.02–0.78, p=0.010), 6 months (p=0.005) and 12 months (p=0.004), and lower respiratory tract infection through 12 months (OR 0.45, 95% CI 0.21– 0.95, p=0.027).
• Infants receiving EF were also less likely to receive antipyretics through 4 months (OR 0.44, 95% CI 0.20-0.98, p=0.032), and antibiotics through 6 months (OR 0.53, 95% CI 0.27-1.02, p=0.047) and 12 months (p=0.016).
Of course we have to be careful with the interpretation of these results since they were secondary endpoints of the study.
Fecal community types in infants at 3 months
If you put all data of the enrolled infants together you can
find 3 types of fecal community type (FCT), namely
• FCT A: low bifidobacteria, high enterobacteriaceae/
lachnospiraceae
• FCT B: high bifidobacteria and low others
• FCT C: medium bifidobacteria and also the others
When we now looked in the three groups in our study (EF, CF, BF) we see again a big difference between the breastfed and formula fed. But it seems that in the test group (EF) the FCT B was higher and the FCT C was lower than in the control group (CF). (Chart 5)
And if we compare that to the antibiotic use it looked that especially the FCT C was more prominent in the group that was receiving the antibiotics. All those data seem to go into the same direction and are concordant to the in vitro data.
But this of course need further research in order to confirm these results.
Conclusion
- First clinical data demonstrate that synthetic HMOs are safe and well tolerated (structurally identical to those found in human milk)
- First clinical data show that formula with 2’FL + LNnT promotes the Fecal
Community Type B seen in breastfed infants
-
The reported reduced likelihood of antibiotic use with 2’FL + LNnT may be linked to the Fecal Community Types
- After these first promising health outcomes, further studies are needed to establish efficacy
