The Biomechanics of Breastfeeding: Bridging the Gap between Engineering-Based Studies and Clinical Practice
Abstract
Currently accepted “best practice” for managing breastfeeding effectively (WHO/UNICEF) is largely based on a historical view of how babies remove milk from the breast, which had persisted for several centuries. The collective wisdom was verified by imaging studies in the 1950s and 1980s, to reach a consensus view – clinical management principles, based on such research, have proved highly effective. Over the past decade, the mechanics of suckling, and how the baby removes milk from the breast, have been revisited, using modern imaging technology and by the application of engineering-based techniques, which seek to develop explanatory models of how suckling works. While the imaging studies have caused us to expand our view of the process, the engineering-based models have proved somewhat contradictory, tending to undermine the new consensus. Such models are complex, mathematically difficult to evaluate, and without simple lessons by which clinicians/practitioners can update their practice. This presentation will seek to demonstrate the current agreement between imaging studies, and elucidate recent engineering- based models of milk extraction, to achieve a fresh consensus – a “revised suckling physiology.” Certain limitations of the engineering-based models will be addressed, showing why they do not yet provide a definitive explanation of how babies remove milk from the breast. The encouraging news, however, is that current “best practice” for breastfeeding does not need to be updated; in fact, a new conclusion indicates that the guiding principles are even more relevant than before.
Introduction
For the best part of four centuries, the medical world was secure in its view of how babies fed and removed milk from the breast – the terms “sucking” and “suckling” became mutually replaceable, even though they describe separate processes. Two commonly accepted facts remain today: first, the baby generates high levels of suction pressure in the oral cavity, so that any object placed in a baby’s mouth (bottle-teat, finger, pacifier) cannot easily be removed. Second, the baby’s tongue moves in a wave-like manner, with positive pressure being exerted rhythmically by the dorsum of the tongue surface to the underside of the nipple/breast complex held in the baby’s mouth; this is regarded as a type feature of the baby, taken to indicate its neurodevelopmental maturity.
The latter tongue movements, originally identified by practical observation [1–3], were later visualized using various techniques including cineradiography [4], 2D ultrasound [5, 6], direct filming [7] and, most recently, 3D ultrasound [8]. All such methods were unambiguous in observing peristaltic tongue movements (PTMs), on the basis of which it was assumed that they played a role in expressing milk from the breast; we have since confirmed that this collective view is essentially correct [9, 10].
Two studies, principally Eishima [7] and Geddes et al. [11], identified a novel feature of infant feeding, involving a localized drawing down of the central region of the tongue, adjacent to the nipple tip. To this movement was imputed the ability to generate increased (added) suction at the nipple surface, claimed to play a predominant role in milk extraction from the breast; subsequent studies have extended and elaborated on these claims [12–14]. We can similarly con- firm that these authors are correct in their observations, and in their proposition that the action aids milk extraction; nonetheless, some vital caveats need to be considered when evaluating the full validity of their claims.
What We Already Know
Seven principal forces are present and active during breastfeeding, the first three affect the pressure of milk within the breast; all but one is active in milk transfer, while one plays a key role in retaining the breast within the baby’s mouth.
(1) Atmospheric pressure is an important force in the process, although it is eclipsed by the positive pressure created by (2) the mother’s “let-down” or milk ejection reflex (MER). The MER creates phasic (intermittent) increases in positive pressure in the milk held within the breast, while also causing the milk ducts to dilate, so providing less resistance to the flow of milk to the nipple surface.
Because breastfeeding is such a highly dynamic form of milk extraction, atmospheric pressure is more likely to play a role in milk extraction by breast pump (being less dynamic). These two forces constitute one side of an active pressure gradient.
A less obvious process creating positive pressure in the breast is (3) the compressive pressure of the baby’s lips against the breast, pressing cyclically on the breast surface surrounding the ducts during feeding. Its role in milk extraction is not dissimilar to that of the flanged cone of a breast pump, pressing against the breast; an awareness of this subsidiary process has largely arisen from studies of breast pumping. A well-attached baby, making a wide flange at the breast, will capitalize on this, while also taking a large mouthful of breast tissue.
Within the baby’s mouth, creating the other side of the active pressure gradient, is (4) intense negative suction pressure created by the baby cyclically lowering the rear surface of its tongue. This is responsible for generating baseline suction pressure which both draws the breast into the baby’s mouth and retains it throughout the feed. This force is unstable, however, as any milk issuing from the breast into the oral cavity negates it, making it necessary for it to be reapplied in a cyclical manner throughout feeding.
These four principal forces are necessary and sufficient for a breast pump (electric or manual) to create adequate milk removal from the breast. The cyclical application of negative suction pressure at the nipple surface (aided by the three other factors) is adequate for sustaining milk collection from the breast.
The two unique features which the baby brings to the process are: (5) the compressive action of the baby’s jaws (and gums), and (6) PTMs applying retro- grade waves of positive pressure to the underside of nipple surface. The peristaltic action of the baby’s tongue is obligate, playing the primary role in both milk transfer and expelling the milk bolus into the oropharynx for swallowing. The action of these two forces alone is not dissimilar to hand expression of the breast, which requires no negative suction pressure to remove milk. The fingers press into the breast at the base of the ducts, in a similar action to the baby’s jaws, and the opposed fingers are drawn towards the nipple end to express milk; this emulates the peristaltic action of the baby’s tongue. The role of the baby’s jaws should never be overlooked, as they effectively “gate” the release of milk, letting it enter the milk ducts, lying within the nipple/breast teat complex, in packaged bundles, rather than as a continuous outflow of milk from the breast.
Accordingly, there are two sets of forces – the first four alone are capable of milk extraction, and are employed specifically when a breast pump is used to extract milk. The next two forces alone are capable of milk expression from the breast, in the absence of the other forces. They are akin to “pump extraction” and “hand expression” and use entirely different modes of action, yet both are effective for expressing/extracting milk from the breast (just as “hand milking” and “machine milking” are equally effective with dairy animals). The essential beauty of mammalian suckling is that these separate forces combine in the baby’s mouth, and are most likely to be acting synergistically to remove milk in the most efficient way possible (the same is true of feeding by most dairy animals studied (sheep and goats) [15], and by pigs [16]).
Two of these forces are well illustrated in a recent study by Grassi et al. [17] who fitted two sensors to a pacifier, one monitoring positive pressure from the jaws and gums, and one measuring negative suction pressure within the oral cavity. Their figure (Fig. 1) shows the negative suction profile and positive pres- sure profile superimposed on each other, illustrating the relative scale and timing of these pressure forces at play during sucking on a pacifier.
Negative pressure (–150 to –200 mbar) is some 3–4 times greater in intensity than positive pressure (50 mbar). Negative suction pressure starts being generated while the jaws are relaxed/open, thereby ensuring the “teat” (or nipple/ breast complex) is drawn into the oral cavity. Positive pressure by the jaws causes the gums to clamp on the base of the “teat,” thereby retaining it in the mouth. Shortly after jaw pressure reaches its peak, and starts to decline, negative pres- sure begins being regenerated. This is caused by lowering of the rear surface of the tongue, which, itself, is the end phase of the peristaltic wave as it completes its traverse of the oral cavity.
This is a key demonstration that even when feeding on an artificial teat (pacifier), the same natural forces are at play, despite them no longer having the normal function they would during breastfeeding.
The final force (7) is localized drawing down of the tongue surface adjacent to the nipple tip [7], the existence of which, and its role in milk removal, has only been confirmed in the past decade [11]. Unlike PTMs, this action is not obligate, but appears more facultative or opportunistic, only being superimposed on PTMs for a proportion of the time spent sucking. These localized depressions of the tongue surface are deployed at particular times during most feeds. Nonetheless, while they are ubiquitous, they cannot exist in isolation from PTMs; recent evidence (below) indicates they are generated by the same process. Their effect is to produce increased or added suction pressure local to the nipple surface; in all likelihood, this facilitates or enhances milk extraction. The phrase extractive tongue depressions (ETDs) will be used to refer to these, in view of their assumed function. While the appearance of ETDs is difficult to predict, they are regularly associated with times of high milk outflow from the nipple. This association between peak negative suction pressure and “peak milk flow” was first revealed by Geddes et al. [11], who proposed that ETDs play a predominant role in milk extraction (in very much the same way as a breast pump would). The ability to detect “peak milk flow” was visually based on the movement of “echogenic flecks” in the space just beyond the nipple tip (stated to be “milk fat”). My personal belief is that these visual markers of milk flow are in fact evidence of “stable cavitation” [18], caused by microbubbles of carbon dioxide being drawn out of solution by the high negative suction pressure, usually being reabsorbed before the milk bolus is swallowed (this theory has yet to be tested or confirmed).
Fig. 1. Example of output from sensors. Right: 60 s selection of the signals acquired during an experimental test. Left: zoom of a single burst and extracted parameters [17].
Engineering-Based Approaches to Modelling Milk Removal from the Breast
The evidence gleaned from multiple imaging studies has since been expanded by employing engineering-based models of the milk duct structure of the breast, and the baby’s sucking pattern, in order to generate theoretical data on milk flow, for comparison with real clinical data.
The first substantive attempt to develop a mathematical model of milk extraction during breastfeeding was undertaken by Zoppou et al. [19]. Drawing on knowledge current at the time, they compared the action of a breast pump, which used a cyclic pattern of suction, with that of a baby using both suction and expression. Their theoretical model caused them to conclude that there was an optimal time during the suck cycle to apply a compressive force, which increased milk flow over that produced by suction alone. Given their conclusion, it is somewhat surprising that recent models have not included a compressive component.
Two recent studies of milk removal from the breast have adopted an engineering-based approach [20, 21]. These studies have sought to create mathematical models which simulate the dynamic relationship between suction generated in the baby’s oral cavity, and the milk-filled duct system of the breast [20, 21]; both studies use data recorded directly during breastfeeding. They are elegant, complex, and sophisticated, although it can prove difficult to evaluate them fully, in order to determine if any of the assumptions made might create erroneous conclusions. Modelling of the milk duct system of the breast is complex and will be bypassed in the discussion below, assuming them to be essentially accurate – that of Mortazavi et al. [21] is claimed to be more elaborate.
Elad et al. [20] have made some expansive claims about their work, but critical analysis of their study suggests some shortcomings. One noteworthy beneficial feature of their study is that their data analysis process allows them to use the hard palate as a register for movements of the tongue (Fig. 2). A relative weakness, in contrast, is that their data are derived from review of a relatively small selection of ultrasound images.
In brief, their methodology is as follows: 5–8 points are digitized on the hard palate (this is a manual process), to which a smooth curve is fitted by interpolation (red line in their Fig. 1A); the same process is applied to the tongue surface (green line). A set of tracings of the hard palate is collected for 4–6 suck cycles, which are then aligned with reference to the Hard-Soft Palate Junction, so that movement of the tongue relative to the hard palate can be visualized (identified as Fig 1A in their figure (Fig. 2A)).
Superimposed on this image (their Fig. 1C (Fig. 2A)) is a set of 28 equally spaced radiating lines (referred to as “polar coordinates,” numbered 1–27 in the figure), which radiate out from the scan head to above the hard and soft palate. The movement of the tongue surface is then plotted along every 5th or 6th polar coordinate, enabling the time lag, relative to the preceding focal co-ordinate to be visualized.
One apparent limitation of their approach is that 28 polar coordinates do not fully encompass the whole of the oral cavity. This might be regarded as a trivial issue, but the full passage of a suck across the oral cavity determines the overall suck duration, so that more lines would be required, up to at least 36, in order to embrace a full suckling action, including the prepharyngeal phase of swallowing. Evaluating movement of the tongue surface relative to the hard palate, across four focal polar coordinates – #8, #13, #17, and #22 (illustrated), shows evidence of a phase shift between these separated lines (their Fig. 1E (Fig. 2B)).
In marked contrast, the time shift between ALL of the first 8 polar coordinates is evaluated and no phase shift is seen between individual lines. Based on their Fig. 1D (reproduced in my Fig. 2C), they assert that there is no phase shift between the lines, indicating that the “anterior tongue moves as a rigid body … ruling out the hypothesis of a peristaltic squeezing of the nipple” [20].
Personally, this line of argument appears misleading to me. Certainly, movement along the first three coordinates closest to the mandible (#1–3) is likely to be determined largely by up/down movement of the jaw, but beyond this point, there is evidence of propagation of a peristaltic wave from as early as polar co- ordinate #4, right through to #28.
The study by Monaci and Woolridge [22] merits discussion in this context, as it adopted a similar approach, but used signal processing techniques to analyze ultrasound records in real-time, thereby generating fully objective, automated results. They arbitrarily divided the oral cavity into three, spatially separated, non-overlapping sectors, equating to: (1) the anterior sector of the oral cavity, including nipple and front part of tongue (excluding lower jaw); (2) the middle sector of the oral cavity, comprising the mid-surface of the tongue and the space at the tip of the nipple in which milk accumulates; and (3) the posterior sector, comprising the oropharynx, where swallowing can be detected (Fig. 3).
These authors also examined the time shift between movements in each of these three areas. Ultrasound recordings from 29 mother/baby pairs (46 complete breastfeeds) were analyzed, although analysis was restricted to those periods when active sucking was taking place. Nonetheless, over 1 million frames of active sucking were analyzed in real-time by this technique.
If movement occurred in sector 1 first, a negative phase shift was recorded relative to sector 2; a zero phase shift indicated an absence of a phase shift be- tween sectors 1 and 2; while a positive phase shift indicated movement in sector 2 preceded that in sector 1. In practice, this was caused by the movement in sec- tor 2 being of larger amplitude than that in sector 1 and was commonly evidence for the presence of an ETD being inserted (i.e., an “added” suction element being superimposed on a peristaltic wave).
Figure 4 encompasses approximately 70 sucks, and illustrates the transition from a period of almost pure “peristaltic” sucking (frames 0–1,000), to a “vacuum” phase where ETDs predominate (alongside PTMs) (frames 1,000–1,500).
The “movement detection rectangles” were manually drawn, so needed to be redrawn when there was movement artefact. Despite this limitation, the signal processing approach was applied to all 46 breastfeeding episodes, totaling 16 h of recording. Overall results of the analysis are shown in Table 1.1 PTMs were present throughout active sucking (100%), being: highly conspicuous for 78% of feeding, and predominating for over half of the time spent feeding, to the exclusion of ETDs (“suction/vacuum”). For a substantial period of feeding (27.5%), both PTMs and ETDS were equally visible, with no one method predominating over the other. For 22% of feeding, the added suction elements (ETDs) appeared to predominate. This analysis shows that ETDs [7, 12] were observable for roughly half of the time spent feeding.
The same authors used a second analytical technique involving automated mapping of the contour of the surface of the tongue. This technique is capable of showing the progression, across successive frames, of a peristaltic wave from the anterior to posterior of the oral cavity. In Figure 5, the peristaltic wave is seen rising in amplitude, then declining, as it transitions (left to right) from the front to the back of the oral cavity.
One final piece of evidence supplied by this latter technique is that when an ETD is generated, the space created is generated as part of the standard peristaltic wave, as it progresses across the zone where the ETD appears; it is both opened at its leading edge initially, then closed off again from its anterior edge (Fig. 6).
The two pictures show the contour of the dorsum of the tongue, which is automatically tracked (using the purpose-built software); the tongue outline is compressed left to right in this figure. The dotted line shows the tongue’s outline in the current frame, while the continuous line shows that in the previous frame. The circle circumscribes the mid-section of the baby’s tongue where the ETD is generated.
The upper picture shows the precise moment the ETD starts to be generated, as the continuous line shows an absence of any indentation, while the dotted line peels away markedly to create an indentation (marked with an X), representing the start of the formation of an ETD “pocket.” In the lower picture, just four frames later, the ETD “pocket” is clear in the continuous line, and it is just starting to be closed off again, from the front (marked with a Y). This is the clearest evidence to date that added suction elements (ETDs) are created by the same core peristaltic process.
Returning to the most recent engineering-based study, Mortazavi et al. [21] created a complex model of the milk duct system of the breast, which they then combined with directly measured suction pressure data (Fig. 7) (from several babies), to define the parameter boundaries of the mathematical model. Modelled milk output was then compared with clinical data on milk transfer for a single baby.
No data were collected on positive stripping pressure, so axiomatically, any such element was excluded from the model, despite it being an explicit component of one of the key studies they cited [19]. Any theoretical model which only assumes that the baby behaves like a mechanical suction pump is likely either to verify that presumption [20], or find that it is inadequate to explain clinical data on milk transfer [21].
In order to use sucking data in their model as parameter boundaries, sucking profiles were transformed into single harmonic, sinusoidal waveforms, seemingly all with a periodic frequency approaching 1 Hz (1 suck/s) (Fig. 8). The need to simplify natural data for incorporation into their model was no doubt necessary, but this constrains the baby’s sucking pattern to even more closely resemble an electric breast pump.
Their theoretical model simulated milk transfer by one baby, which was then compared with clinical data on intake by that baby. Based on this, the authors were forced to conclude that either sucking pressure alone, or total feed duration, did not account for: (a) the volume of milk removed, (b) the flow rate per unit time, or (c) the flow rate per suck.
This finding is unsurprising as it agrees with that of an earlier detailed study of the parameters of sucking pressure during breastfeeding [23], which was unable to find any association between suction and the 58% difference in intake between the first and second breast. Additionally, several authors have shown an inverse relationship between sucking pressure and milk flow during bottle-feeds: the greater the resistance to milk flow caused by teat hole size, the greater the pressure exerted by the baby to remove milk [15].
A range of other factors are believed to explain the difference in milk intake between the first and second breast, including the “mother’s physiological response to sucking” [21], although no consideration appears to have been given to the fact that satiation by the baby can most commonly be observed when feeding from the second breast [24], and/or that less milk is available from the second breast as a result of the tendency to start breastfeeds on the breast offered last at the previous feed.
Both of the engineering-based theoretical models discussed above [20, 21] projected milk flow to be 1.85–3 times greater than measured intake by the baby, despite using many fewer branching ductal milk lobes than are naturally found in the lactating breast (the 5-lobe model [21] produced less of a discrepancy than the 2-lobe model [20]). Seeking to explain why milk flow was slower in reality, Mortazavi et al. [21] concluded that resistance to milk flow was great- er than predicted in their model. They concluded this was likely to have been caused by factors including: the “deformed region of the areola-nipple,” a “reduction of ducts cross-sectional area,” and/or “elasticity of the tissue.” More generally, they alluded to other parameters, which included: “suckling” (mouthing or chewing movements were otherwise ignored), swallowing, and “breathing interruptions” (coordinating swallows with breathing may retard the rate of milk acquisition).
Fig. 2. A Figure taken from Elad et al. [20] – a full description is contained in the text.
B, C After Elad et al. [10], highlighted enlargement of their their Fig. 1C–E.
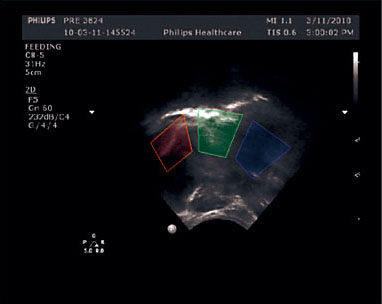
Fig. 3. Frame of ultra- sound recording showing three user-applied rectangles, in which movements can be automatically tracked and compared
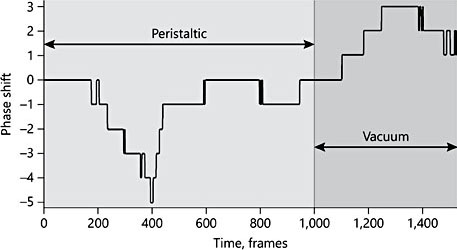
Fig. 4. Section of analysis over a time frame of 60 s, embracing 70 sucks, which captures the transition from one style of suckling to another.
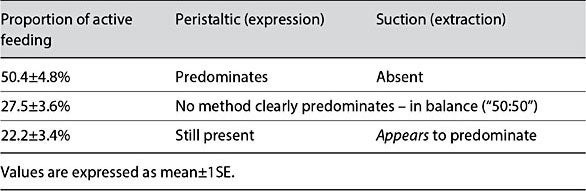
Table 1. Automated analysis of ultrasound recordings, exploring the phase shift in movement between the anterior and mid-sections of the oral cavity
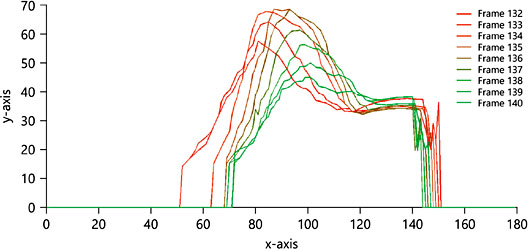
Fig. 5. This figure shows the progression of a peristaltic wave from left (anterior) to right (posterior), across nine consecutive frames.
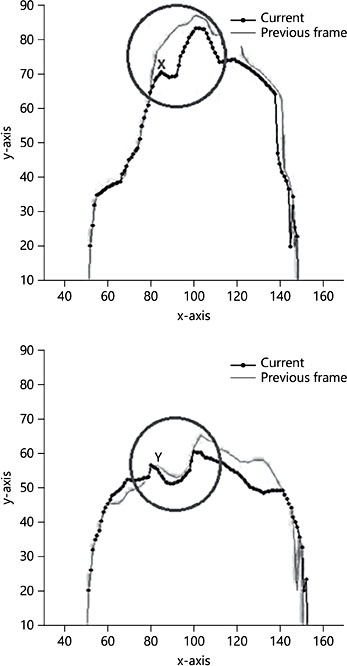
Fig. 6. This figure shows a localized added suck or extractive tongue depression, which is created from the front backwards. Four frames later, it is closed off again from the front back- wards.
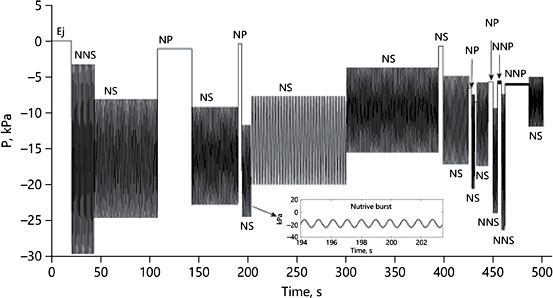
Fig. 7. Phases of natural suckling (by 1 infant) transformed to a sequence of standardized sinusoidal waveforms (see inset). From Mortazavi et al. [21].
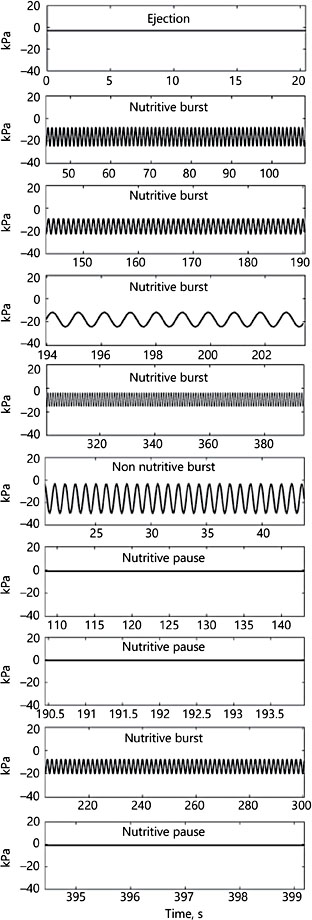
Fig. 8. A selection of pres- sure profiles enlarged from Figure 7. The rate of nutritive sucking for all profiles is 0.92 sucks/s; the non-nutritive rate is not statistically different at 0.95 sucks/s.
Validity of the Engineering-Based Mathematical Models
It is axiomatic that a mathematical model is only as strong as the number of assumptions on which it is based. Based on the methods reported in the development of these models, it is possible to identify several false assumptions which have been made, and incorporated into the models.
Addressing the study by Elad et al. [20] first, one of their key conclusions (from the technique shown in Fig. 2A–C) is that milk removal from the breast is caused by the rigid up/down movement of the baby’s jaws (impacting on the base of the nipple), combined with intraoral negative suction pressure. As a con- sequence, their model is based solely on the action of negative suction pressure, without any consideration of the possible role for the positive pressure wave created by the dorsum of the baby’s tongue. It should therefore come as no surprise that their model predicts that negative suction pressure alone can fully account for milk removal from the breast; essentially, theirs is more a kinetic model of how a mechanical breast pump works.
A key quality issue, likely to affect the validity of modelling studies, is the size of the sample on which any model is based. In the study by Elad et al. [20], 9 subjects, varying in age from 11 to 150 days were included, with a maximum of 15 s of sucking recorded. From these records, “four to six sucking cycles (i.e., about 150 frames) were selected for the analysis of tongue motion.” On the basis that six suck cycles last approximately 6 s, this signifies that data from a total of 54 s of feeding were used to generate the mathematical model. This may be a reason why Elad et al. [20] did not detect, or include in their analysis, the type of tongue movement described by Geddes and colleagues [11–14].
Certain assumptions may also be made to make a model less mathematically complex to compute. Mortazavi et al. [21] state that, in their study, the milk ducts are “assumed to be rigid.” An unstated corollary to this will be that the duct openings are also assumed to be rigid, being held open (patent) throughout the feed. This is recognized as a false assumption, as, in practice, the milk ducts are highly flexible and collapsible; it would not be easy to predict how they would behave dynamically under the combination of both positive pressure from the tongue and negative suction pressure from the oral cavity. Their model also assumes that negative suction pressure plays the sole role in milk removal. Perhaps as a consequence of this, a comparison of simulated data with clinical data from the same baby forces them to conclude that “suction pressure alone cannot account for milk removal from the breast” (the probable factors were discussed above).
Key Physiological Features Not Included in Models
A major physiological fact is overlooked by both these models, however, which is that the baby’s jaws repeatedly compress the nipple-breast/teat complex at its base, at the start of the suck cycle, and do so cyclically throughout the feed. This pressure (approx. 37.5 mm Hg) is likely to occlude the milk ducts with each suck, so it is not appropriate to assume that milk is drawn directly from the breast into the baby’s mouth, on the assumption that the milk ducts remain patent throughout. The milk-filled duct system of the mother’s breast represents a pressure gradient, confluent with the baby’s mouth, which is active at the onset of feeding. Hypothetically, this pressure gradient could ensure continuous movement of milk from within the breast into the baby’s mouth. At the start of the suck cycle, however, with closure of the baby’s jaws, it is no longer active and will only be- come active again at the end of the suck cycle, when the baby’s jaws reopen, and the teat ducts refill with milk from the breast.
This jaw closure, which causes the pressure gradient to be deactivated, persists for 75–80% of the suck cycle; so the pressure gradient cannot be characterized as being active throughout feeding. This is perhaps the biggest limitation of the two engineering-based models published to date, making them approximate much better to how a mechanical breast pump works. Neither provides a satisfactory theoretical explanation of how breastfeeding works (or of manual breast expression for that matter). Further concern should be raised over the assumption that the milk duct apertures remain open throughout; this is unlikely given the close approximation of the nipple to the soft tissues of the baby’s mouth, and the high suction pressures generated within the oral cavity.
Which Force Is Primary in Causing Milk Removal from the Breast?
As suggested above, the active pressure gradient could make it possible for milk to be delivered continuously from the breast to the baby’s mouth, were it not for the “gating” effect of the baby’s jaws. Accordingly, the milk available on each suck is limited to that captured in the milk ducts lying within the baby’s mouth; milk cannot be extracted directly from the breast. A further fact, which should not be overlooked, is that the milk duct openings are very much narrower (by up to 50 times) than the dilated milk-filled ducts leading to them. So, an essential corollary to the question above is: “What force is responsible for opening the duct ends?”
Based on the proposition of Geddes and colleagues [10–14], can it be the case that localised added suction (ETD) at the nipple surface is the force responsible? The answer is likely to be an emphatic “No.” Any level of suction pressure applied outside the nipple surface (if this exceeds the positive milk pressure created by the mother’s MER), is likely to cause collapse of the teat openings. While suction can be transmitted through a fixed aperture, and propagated back along a rigid tube, this cannot occur in the flexible, collapsible milk duct system of the breast. Nipple duct opening, therefore, cannot be achieved from outside the nipple surface.
Instead, this can only be achieved from within, by increasing intra-ductal pressure. This is precisely what the peristaltic tongue movements do. Having captured milk within the milk ducts held in the oral cavity, the peristaltic wave of compression squeezes this milk towards the nipple end; the resulting rise in intra-ductal pressure forces the milk duct ends open. Only when this has happened, might extra-ductal pressure (added intra-oral suction from an ETD) be capable of enhancing either the rate of milk extraction, or the net volume of milk transferred during that suck. The mechanism by which added suction is likely to achieve this is by extending the suck duration, potentially achieving more effective emptying of the ducts.
From this perspective, not only are peristaltic tongue movements (PTMs) the obligate, primary tongue movement, present throughout active sucking, they also appear to be the primary mechanism by which milk is forced towards the duct openings, and out into the baby’s mouth. It may be deduced from this that the efficiency of such a mechanism will depend on the surface area of the nipple- breast “teat” complex lying against the baby’s tongue. In addition, the wider the baby’s mouth is flanged, the better will be its apposition to the breast; resulting in a greater mouthful of breast tissue being taken by the baby. Both these key features will be enhanced by maximising the “positioning” and “attachment” of the baby at the breast.
A Final Piece of Evidence
One final piece of unique evidence comes from a historical study, not from any recent research. In the 1980s, Alan Lucas, then based at the John Radcliffe Hospital in Oxford, came up with the novel idea of measuring milk transfer from a mother to her baby directly, by placing a flow transducer between them, housed in the tip of a latex nipple shield [25]. The research team (Bio-Engineering Unit) developed a Doppler ultrasound flow transducer which insonated an area of parallel milk flow, created as breast milk passed through the transducer body. This technique provides completely unique views of instantaneous milk transfer during suckling [26]; I have been able to revisit a proportion of the original milk flow traces, undertaking some fresh analysis of them, in an attempt to resolve some the issues emerging from the “revised suckling physiology” above.
If, as Geddes et al. [11] assert, added suction (ETDs) is the predominant force in milk removal from the breast, then one would be likely to observe a “mid- suck” peak in milk flow, with relatively little milk flow either side of this. In practice, this is not the case – peak milk flow is invariably seen early in the suck cycle (first 20%), tailing off towards the end (Fig. 9). In many sucks, following an early high amplitude flow, a later more attenuated phase of irregular milk flow may be observed; this is most conspicuous in sucks of longer duration. These sucks are most likely to be those which include an ETD, which were shown by Eishima [7] to extend the suck duration.
More commonly, the two phases of milk flow grade into each other, so first we have a high-amplitude, short-duration flow, followed by a lower-amplitude, longer-duration flow. The net contribution each of these makes to milk transfer may be the same, although it is important to remember that the secondary peak of milk flow may be absent in a large proportion of sucks. This novel source of information about milk flow during suckling suggests that baseline suction and PTMs are uniquely responsible for initiating and maintaining milk flow on each and every suck. When ETDs are superimposed on the incipient rhythm, they appear to enhance milk flow, mainly by sustaining it over a longer duration.
This unique insight into the process of milk transfer has been provided, not by new engineering-based models, but by a much earlier piece of research. Nonetheless, we have only recently been able to explain fully the complex shape of the milk flow profile in light of the evidence that both PTMs and ETDs coexist during breastfeeding, demonstrating that the baby both suckles and sucks milk from the breast.

Fig. 9. Section of a milk flow trace obtained with a Doppler ultrasound flow transducer. Suck duration is shown in the box around each one, so, in terms of whether they are long (L) or short (S), this series of seven sucks is L-S-S-S-L-S-S.
Clinical Implications of the “Revised Suckling Physiology”
Based on the evidence presented above, it is reassuring to learn that the standard tenets of good breastfeeding technique remain as true today as when they were first proposed [27, 28]. Enhancing the Positioning and Attachment of the baby at the breast will have the specific benefit of maximizing mouth:breast apposition. The greater the mouthful of breast tissue the baby takes, the further it will extend into the oral cavity, so providing a greater opportunity for the dorsum of the baby’s tongue to compress the underside of the teat. Explicitly, this will maximize the baby’s ability to amplify intraductal pressure during the suck, thereby boosting milk expression. Only when the duct ends have been opened by pressure from within, will added suction (ETDs) be capable of enhancing milk flow. No intervention has yet been identified which allows the level of suction that the baby produces to be modified (although the faster the rate of milk flow, the less suction pressure is likely to be applied). Accordingly, the basic tenets of Positioning and Attachment would apply here also.
References
- 1 Cooper AP: On the Anatomy of the Breast. London, Longman, Orme, Green, Brown & Longmans, 1840, vol I, II.
- 2 Darwin C: A biographical sketch of an infant (1875). Dev Med Child Neurol 1971; 13(s24):3–8.
- 3 Morris D: Babywatching. New York, Crown, 1991.
- 4 Ardran GM, Kemp FH, Lind J: A cineradiographic study of bottle feeding. Br J Radiol 1958;31:11–22.
- 5 Smith WL, Erenberg A, Nowak A, Franken EA: Physiology of sucking in the normal term infant using real-time US. Radiology 1985; 156:379–381.
- 6 Weber F, Woolridge MW, Baum JD: An ultrasonographic study of the organization of sucking and swallowing by newborn infants. Dev Med Child Neurol 1986;28:19–24.
- 7 Eishima K: The analysis of sucking behaviour in newborn infants. Early Hum Dev 1991;27: 163–173.
- 8 Burton P, Ding J, McDonald D, Fewtrell MS: Real-time 3D ultrasound imaging of infant tongue movements during breast-feeding. Early Hum Dev 2013;89:635–641.
- 9 Woolridge MW: The mechanics of breast- feeding revised: new insights into how babies feed provided by fresh ultrasound studies. 5th Europaediatr Congr, Vienna, June 2012.
- 10 Woolridge MW: The mechanics of breast- feeding: does the baby suck or suckle? Findings from 2D ultrasound studies. 20th Annu Int Meet Acad Breastfeed Med, Los Angeles, October 2015.
- 11 Geddes DT, Kent JC, Mitoulas LR, Hartmann PE: Tongue movement and intra-oral vacuum in breastfeeding infants. Early Hum Dev 2008;84:471–477.
- 12 Geddes DT, Sakalidis VS, Hepworth AR, et al: Tongue movement and intra-oral vacuum of term infants during breastfeeding and feeding from an experimental teat that re- leased milk under vacuum only. Early Hum Dev 2012;88:443–449.
- 13 Sakalidis VS, Williams TM, Garbin CP, et al: Ultrasound imaging of infant sucking dynamics during the establishment of lactation. J Hum Lact 2013;29:205–213.
- 14 Sakalidis VS, Hepworth AR, Hartmann PE, Geddes D: Ultrasound imaging of infant sucking dynamics during the establishment of lactation. J Hum Lact 2013;29:205–213.
- 15 Ardran GM, Kemp FH: A correlation be- tween suckling pressures and the movements of the tongue. Acta Paediatr 1959;48:261– 272.
- 16 Thexton A, Crompton A, Owerkowicz T, German R: Correlation between intraoral pressures and tongue movements in the suck- ling pig. Arch Oral Biol 2004;49:567–575.
- 17 Grassi A, Cecchi F, Sgherri G, et al: Sensorized pacifier to evaluate non-nutritive sucking in newborns. Med Eng Phys 2016;38: 398–402.
- 18 Unsworth A, Dowson D, Wright V: Cracking joints. A bioengineering study of cavitation in the metacarpophalangeal joint. Ann Rheum Dis 1971;30:348–358.
- 19 Zoppou C, Barry SI, Mercer GN: Dynamics of human milk extraction: a comparative study of breast feeding and breast pumping. Bull Math Biol 1997;59:953–973.
- 20 Elad D, Kozlovsky P, Blum O, et al: Biomechanics of milk extraction during breast- feeding. Proc Natl Acad Sci USA 2014;111: 5230–5235.
- 21 Mortazavi SN, Geddes D, Hassanipour F: Lactation in the human breast from a fluid dynamics point of view. J Biomech Eng 2017; 139:011009.
- 22 Monaci G, Woolridge MW: Ultrasound video analysis for understanding infant breastfeeding. Proc 18th IEEE Int Conf Image Process (ICIP), September 2011. DOI: 10.1109/ ICIP.2011.6115802.
- 23 Prieto CR, Cardenas H, Salvatierra AM, et al: Sucking pressure and its relationship to milk transfer during breastfeeding in humans. J Reprod Fertil 1996;108:69–74.
- 24 Drewett RF, Woolridge MW: Milk taken by human babies from the first and second breast. Physiol Behav 1981;26:327–329.
- 25 How TV, Ashmore MP, Rolfe P, et al: A Doppler ultrasound technique for measuring human milk flow. J Med Eng Technol 1979: 3:66–71.
- 26 Woolridge MW, How TV, Drewett RF, et al: A method for the continuous measurement of milk intake at a feed in breast-fed babies. Early Hum Dev 1982;6:365–373.
- 27 Chalmers I, Enkin M, Keirse JMC: Effective Care in Pregnancy and Childbirth. Oxford, Oxford University Press, 1989.
- 28 Renfrew MJ, Woolridge MW, Ross McGill H: Enabling Women to Breastfeed: A Review of Practices Which Promote or Inhibit Breast- feeding – With Evidence-Based Guidance for Practice. London, The Stationery Office, 2000.