Metabolic Regulation of Pre- and Postnatal Growth
Introduction
Growth characteristics during periods of early developmental plasticity are closely linked with later health outcomes, such as physical and cognitive performance and the risk of later disease [1, 2]. Traditionally, pediatric medicine has focused on the adverse effects of undernutrition on reduced growth and on later outcomes such as adult stature, cognitive ability, educational achievements, productivity and income, and life expectancy [3, 4]. More recently, childhood overnutrition and overweight have also become major concerns since they are even more prevalent globally than undernutrition [5–8]. Ample evidence now links high early weight gain in infancy and the second year of life to later risks of obesity, adiposity, and associated noncommunicable diseases (NCD) such as type 2 diabetes, hypertension, cardiovascular diseases, and asthma [9, 10]. Ac- cordingly, the World Health Organization emphasized the need to enhance in- vestments in obesity prevention approaches in early life when particularly large benefits are achievable [11].
Infant weight gain is thought to be determined by both nonmodifiable and modifiable predictors, including genetic and epigenetic characteristics, parental size, infant sex, psychosocial and other environmental conditions, infections and inflammation, hormonal regulation, and not least by nutrition and metabolism. Great preventive opportunities appear to exist through optimizing nutrition in early life, which can modify metabolic and endocrine responses, growth trajectories, body composition, and related health outcomes. Detailed under- standing of related metabolic regulation can guide the design and implementation of targeted preventive strategies. Therefore, we aim to explore metabolic modulators of growth and health outcomes. To do so, we developed and apply clinical targeted metabolomics with a sensitive and precise high-throughput platform. The metabolome is the totality of all small-molecule metabolites (<1,500 Da) in a biological sample or the body, which includes endogenous metabolites such as substrates, intermediates, products of enzyme-mediated metabolic pathways, as well as exogenous chemicals such as drugs, contaminants, food additives, toxins, and microbial products that reflect the composition and metabolic activity of the microbiome. Clinical targeted metabolomics is the study of small-molecule metabolite profiles to characterize biochemical path- ways, physiology, environmental and dietary exposures, and health and disease outcomes. Among all the modern “omics” techniques, metabolomics is closest to the human phenotype: the degree of association to phenotype and health gen- erally increases from genomics, to epigenomics, transcriptomics, proteomics, and finally to metabolomics. We apply high-performance liquid chromatogra- phy (LC) coupled to triple quadrupole mass spectrometry (MS/MS), which enables us to quantify hundreds of molecules in small biological samples, e.g., 50 μL plasma or less [12–18]. The pattern of metabolites determined, which include substrates, intermediates, and products of biological processes, can provide bio- markers of exposures and outcomes, and it can allow insights into underlying metabolic mechanisms. Here, we present examples of the study of metabolic predictors of fetal and infant growth based on recent studies with large sample sizes.
Intrauterine Metabolic Modulation of Growth
It is generally assumed that the fetal supply of glucose and fatty acids (FA) is the dominant metabolic modulator of fetal growth. Fetal glucose and FA supply can be reduced with placental dysfunction and be enhanced with maternal obesity and gestational diabetes. Fetal insulin secretion is thought to respond to the placental substrate and particularly to transplacental glucose supply. We aimed to address the question of whether other metabolites are also predictive of fetal growth, as reflected by infant birth weight, given that only very limited data have been available on the relationship of the cord blood metabolomic profiles to birth weight or later growth. In previous studies characterizing the longitudinal patterns of fasting maternal metabolites during the course of pregnancy among healthy pregnant women, we found plasma concentrations of several essential and nonessential amino acids (AA), long-chain polyunsaturated FA, free carnitine, acetylcarnitine, phosphatidylcholines, and sphingomyelins to decrease significantly with advancing gestation, whereas concentrations of several tricarboxylic acid cycle intermediates and the ketone body β-hydroxybutyrate in- creased [15]. These data led us to hypothesize that other factors in addition to placental energy supply through glucose and total FA may be related to fetal growth.
Therefore, we studied children from the Munich and Bad Honnef study centers of the LISAplus study, a German prospective birth cohort study on the “Influence of Lifestyle-Related Factors on the Development of the Immune System and Allergies in East and West Germany – plus the Influence of Traffic Emissions and Genetics,” which followed children to the age of 15 years [19]. We included 753 children with available cord blood plasma samples and anthropometric data in our study. Approval by the local ethics committees and written parental consent were obtained.
Metabolomic measurements were performed at the Division of Metabolic and Nutritional Medicine, Dr. von Hauner Children’s Hospital, LMU Munich, as previously reported [12], using gas LC with flame ionization detection for FA quantification, and LC-MS/MS with an electrospray ionization source for the analysis of phospholipid molecular species, AA, and other metabolites. Data on birth weight, and weight and height at 2 years of age were obtained from medical records, whereas weight and height at 15 years of age were measured at study center visits or obtained from parental questionnaires. Weight-for-length z- scores at birth and 6 months of age and BMI z-scores at 2 and 15 years were calculated based on WHO growth standards.
After applying our standard quality control procedures, associations were tested using linear regression models adjusted for study center, sex, gestational age, maternal smoking during the third trimester, maternal prepregnancy BMI, maternal weight gain during pregnancy, and maternal education. Sex-stratified analysis was performed for metabolites significantly associated with birth weight [12]. Bonferroni correction for multiple testing was applied to all analyses.
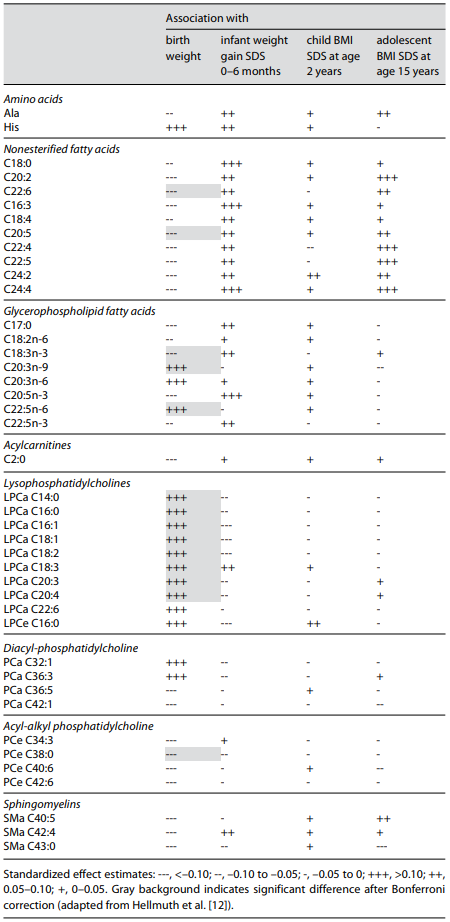
In this study with a large sample size, we found birth weight positively associ- ated with the lysophosphatidylcholines (LPC) C16:1, C18:1, C20:3, C18:2, C20: 4, C14:0, C16:0, C18:3, as well as the glycerophospholipid-FA C20:3n-9 and 22: 5n-6 (Table 1). Birth weight was inversely correlated to the nonesterified FA C22:6 and C20:5, glycerophospholipid-FA C18:3n-3, and acyl-alkyl-linked phosphatidylcholine C38:0. Interestingly, there was a clear sex effect, with closer associations of several metabolites to birthweight in newborn girls than boys. This underlines the importance of considering sex in metabolomic analyses, which also is apparent from sex effects in other studies [16]. Several metabolites, particularly nonesterified FA species, also showed a trend to predicted weight gain from birth to 6 months and to BMI at age 15 years, but with the available sample size these associations did not remain statistically significant after cor- rection for multiple testing [12].
LPC in the blood is derived from cleavage of phosphatidylcholine by phospholipase A2, endothelial lipases, and lecithin-cholesterol acyltransferase, which all liberate FA from the sn-2 (middle) position of the phosphatidylcholine mol- ecule, comprising predominantly the ω-6 PUFA linoleic acid and arachidonic acid, followed by oleic acid. It is tempting to speculate that the documented LPC associations might reflect an intrauterine growth-promoting effect of ω-6 PUFA, which would also be compatible with the other FA associations that we found.
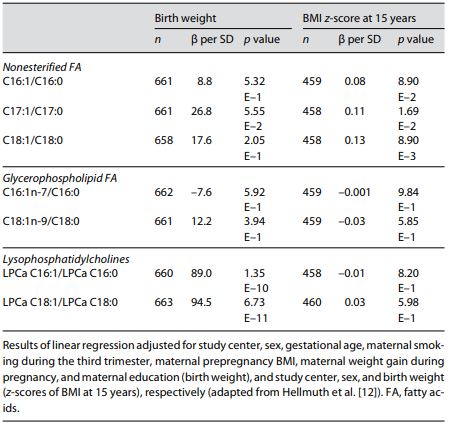
We also calculated ratios reflecting the desaturation index for the enzyme stearoyl-CoA desaturase (SCD-1; also referred to as Δ-9-desaturase). SCD-1 is an enzyme located in the endoplasmic reticulum that mediates the rate-limiting step in the formation of monounsaturated FA (MUFA), such as oleate (18:1) from stearoyl-CoA (18:0) and palmitoleate (16:1) from palmitoyl-CoA (16:0). We found the ratios of LPC 18:1 to 18:0 and 16:1 to 16:0 positively associated with birth weight after Bonferroni correction (Table 2). SCD-1 is a potential key enzyme in the development of obesity promoting lipogenesis rather than oxidation, which results in larger fetal fat deposition and higher birth weight, and alterations in SCD-1 activity have been associated with adult obesity [20].
Table 1. Cord blood metabolites in children participating in the German prospective birth cohort study (LISAplus) that were associated with birth weight, weight gain standard deviation scores (SDS) during the first 6 months, and BMI SDS at ages 2 and 15 years based on a false discovery rate of <5% enzyme in the development of obesity promoting lipogenesis rather than oxidation, which results in larger fetal fat deposition and higher birth weight, and alterations in SCD-1 activity have been associated with adult obesity [20].
Our study shows that cord plasma metabolites, in particular metabolites that reflect intrauterine lipid and FA metabolism, are significantly related to fetal growth and show a nonsignificant trend towards an association with postnatal growth up to adolescence. These data point to potential opportunities of promoting child growth and health through optimized nutrition before and during pregnancy, which should be further explored.
Metabolic Response to Infant Protein Supply and Impact on Growth and Obesity Development
Stimulated by our observation that breastfeeding is associated with a consistent moderate risk reduction in obesity at school age and later in life [21, 22], we ex- plored potential mechanisms for the protective effect of breastfeeding. Consid- ering that the protein supply with conventional infant formula is much higher than in breast milk, but not the average supply of carbohydrates and lipids, we followed the “Early Protein Hypothesis” [23, 24], i.e., the concept that high pro- tein intakes over and above infant metabolic requirements, as provided with conventional infant formulae, induce excessive infant weight gain and increase the risk of later obesity. We could confirm the “Early Protein Hypothesis” in an EU-funded, large, double-blind, randomized, clinical trial that enrolled 1,678 heathy infants after birth at full term [25]. We demonstrated causal effects of infant protein supply on short- and long-term growth by comparing formula feeding in the first year of life either with conventionally high protein or with reduced protein contents. A reduced protein supply, more similar to the intake with breastfeeding, prevented excessive early weight gain [25] and achieved a reduction in mean BMI by 0.51 and a mean 2.6-fold-lower adjusted relative obe- sity risk at school age (6 years) [26]. Along with the promotion of breastfeeding, this is the most powerful strategy for the prevention of childhood obesity known today, and it was promptly implemented in guidelines [27, 28], European infant food legislation (2016) [29], marketed products, and in infant feeding practices. We studied the metabolic response to a higher or lower protein supply in in- fancy to try to elucidate underlying mechanisms [30]. Plasma samples of 764 infants who received formula milk with different protein contents (convention- al high protein, HP: 2.05 g/100 mL; intervention with low protein, LP: 1.25 g/100 mL) or who were breastfed were collected. We determined plasma AA and ac- ylcarnitine concentrations using our LC-MS/MS-based targeted metabolomics platform at the Division of Metabolic and Nutritional Medicine, Dr. von Haun- er Children’s Hospital, LMU Munich. Valid measurements for all metabolites were obtained in 691 infants (breastfed = 163, LP = 263, HP = 265). Approval by the local ethics committees and written parental consent were obtained.
Table 2. Associations of cord blood ratios reflecting the desaturation index of SCD-1 with birth weight and BMI at age 15 years in children participating in the German prospective birth cohort study (LISAplus)
We found 29 metabolites significantly different between the HP and LP for- mula groups (Table 3). In a multivariate approach considering metabolite de- pendencies, 14 metabolites (5 AA and 9 acylcarnitines) were selected by random forest analysis. Taken together, these 14 metabolites contain most of the infor- mation on the difference between the metabolic profiles of infants fed a LP or HP formula. Particularly striking are branched-chain AA (BCAA) and their degradation products, the acylcarnitines C3, C4, C5, C5-OH, and C5:1, which are significantly higher in the HP group (all adjusted p < 3.9 × 10–4) (Table 3). Furthermore, among the 14 metabolites were the essential AA phenylalanine and methionine (higher in HP), as well as the acylcarnitines C5-DC, C6:1, C8:1, C12, and the nonessential AA glutamine (higher in LP). The differences were all significant (adjusted significance level of p < 7.7 × 10–4).
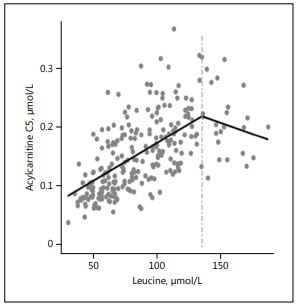
An example of the relationship of the BCAA to their respective degradation products is shown in Figure 1. The breakpoint analysis statistically confirms the existence of a threshold in the degradation rate for isoleucine and leucine. Fur- ther analyses of the relationship of blood urea nitrogen with isoleucine and leu- cine yielded similar results. We interpret these findings as indications for a lim- ited infant capacity of BCAA breakdown via branched-chain α-keto acid dehy- drogenase, which is exceeded with the high plasma AA concentrations that are induced by HP intakes with conventional infant formulas [30]. BCAA may in- duce insulin secretion, β-cell dysfunction, and fat deposition [30], and are thought to upregulate the mTOR (mammalian target of rapamycin) pathway, a possible trigger for promoting protein and fat synthesis as well as cell growth and weight gain. We consider it prudent not to provide protein to infants in amounts which exceed their capacity for metabolizing the supply.
Among the AA generally considered dispensable, tyrosine plasma concentra- tion was markedly elevated. We have previously shown that high plasma tyrosine is associated with elevated insulin concentrations and insulin resistance in obese children before and after weight loss [31]. In fact, in our randomized in- tervention trial, HP supply to infants induced a significantly elevated secretion of the growth factors insulin and IGF-I [32].
Table 3. Selected plasma amino acid (AA) and carnitine/acylcarnitine concentrations with significant group differences in 6-month-old infants from the European Childhood Obesity Project (CHOP) Study, who were ran- domized double blind to a conventional higher protein (HP) or an isoenergetic lower protein (LP) infant for- mula, or were breastfed (BF; nonrandomized)
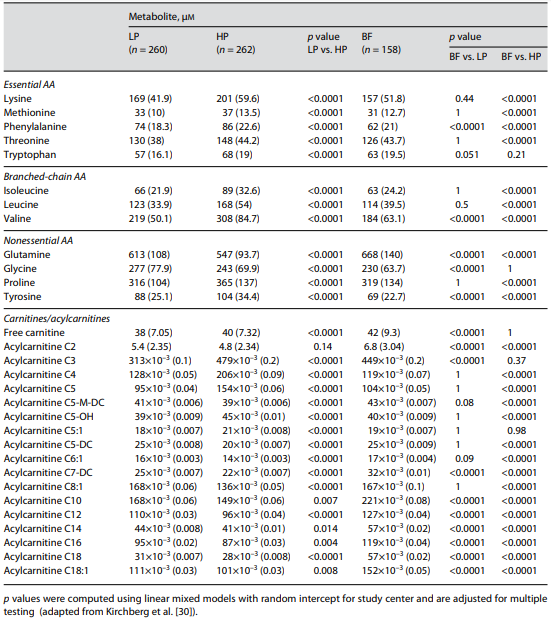
Fig. 1. Scatterplot of plas- ma concentrations of leu- cine versus acylcarnitine C5 in formula-fed infants aged 6 months. With high- er plasma concentration of leucine, the concentration of acylcarnitine C5 increas- es until a breakpoint is reached, indicating that the capacity of leucine conversion is exceeded (drawn from Kirchberg et al. [30]).
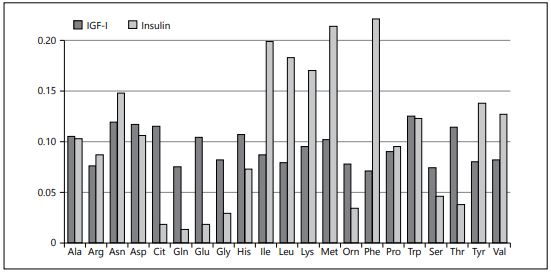
In order to explore potential differential effects of various AA, we performed a path model analysis of data from another double-blind randomized infant feeding trial that compared formulae with different protein quality in healthy infants [33]. We found a generally much greater response of insulin than IGF-I to plasma AA and very different relative effects of individual AA (Fig. 2) [34]. We consider this an important mechanism by which the protein quality pro- vided to infants significantly modifies the energetic efficiency of infant formulae for weight and length gain [35]. Therefore, not only lowering protein quantity but also improving protein quality in infant feeding may provide benefits for growth and health, a concept that deserves further evaluation.
Together, the available data show that plasma metabolites responding to nutritional supply are related to birth weight, postnatal weight gain, and later body weight and obesity risk. Detailed insights into the metabolic regulation of early weight gain may offer tremendous opportunities for more targeted and optimized health prevention through nutritional interventions that promote physiological growth and reduce the risk of later obesity, adiposity, and related non- communicable diseases, including precision nutrition approaches targeting individuals or groups at high risk.
Fig. 2. Variance of different plasma amino acids explained (R2) by IGF-I and insulin con- centrations in 120-day-old infants fed formulae with different protein quality (path mod- el analysis) (modified from Brands et al. [9]).
Acknowledgments
The authors’ work is financially supported by the European Commission, project Early- Nutrition (FP7-289346), MeDALL (FP7-261357), DynaHEALTH (H2020-633595), and LifeCycle (H2020-SC1-2016-RTD), and the European Research Council Advanced Grant META-GROWTH (ERC-2012-AdG 322605). Additional support from the German Ministry of Education and Research (No. 01 GI 0825), the German Research Council (Ko 912/12-1), and the Helmholtz Association is gratefully acknowledged.
Disclosure Statement
None of the authors reports a conflict of interest in relation to the content of this manuscript.
References
- Koletzko B, Brands B, Grote V, et al: Long- term health impact of early nutrition: the power of programming. Ann Nutr Metab 2017;70:161–169.
- Rzehak P, Oddy WH, Mearin ML, et al: Infant feeding and growth trajectory patterns in childhood and body composition in young adulthood. Am J Clin Nutr 2017;106:568–580.
- Chourdakis M, Hecht C, Gerasimidis K, et al: Malnutrition risk in hospitalized children: use of 3 screening tools in a large European popu- lation. Am J Clin Nutr 2016;103:1301–1310.
- Hecht C, Weber M, Grote V, et al: Disease associated malnutrition correlates with length of hospital stay in children. Clin Nutr 2015; 34:53–59.
- Koletzko B, von Kries R, Monasterolo RC, et al: Infant feeding and later obesity risk. Adv Exp Med Biol 2009;646:15–29.
- Rzehak P, Covic M, Saffery R, et al: DNA- methylation and body composition in pre- school children: epigenome-wide-analysis in the European Childhood Obesity Project (CHOP)-Study. Sci Rep 2017;7:14349.
- Symonds ME, Mendez MA, Meltzer HM, et al: Early life nutritional programming of obe- sity: mother-child cohort studies. Ann Nutr Metab 2013;62:137–145.
- NCD-Risk-Factor-Collaboration. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128 · 9 million chil- dren, adolescents, and adults. Lancet 2017; 390:2627–2642.
- Brands B, Demmelmair H, Koletzko B; Early- Nutrition Project: How growth due to infant nutrition influences obesity and later disease risk. Acta Paediatr 2014;103:578–585.
- Flexeder C, Thiering E, von Berg A, et al: Peak weight velocity in infancy is negatively associated with lung function in adolescence. Pediatr Pulmonol 2016;51:147–156.
- Report of the Commission on Ending Child- hood Obesity. Geneva, World Health Organi- zation, 2016.
- Hellmuth C, Uhl O, Standl M, et al: Cord blood metabolome is highly associated with birth weight, but less predictive for later weight development. Obesity Facts 2017;10: 85–100.
- Hellmuth C, Uhl O, Kirchberg FF, et al: Ef- fects of early nutrition on the infant metabo- lome; in Fewtrell MS, Haschke F, Prescott SL (eds): Preventive Aspects of Early Nutrition. Nestlé Nutr Inst Workshop Ser. Vevey/Basel, Nestec/Karger, 2016, vol 85, pp 89–100.
- Uhl O, Fleddermann M, Hellmuth C, et al: Phospholipid species in newborn and 4 month old infants after consumption of dif- ferent formulas or breast milk. PLoS One 2016;29:11:e0162040.
- Lindsay KL, Hellmuth C, Uhl O, et al: Longi- tudinal metabolomic profiling of amino acids and lipids across healthy pregnancy. PLoS One 2015;10:e0145794.
- Rauschert S, Uhl O, Koletzko B, et al: Lipido- mics reveals associations of phospholipids with obesity and insulin resistance in young adults. J Clin Endocrinol Metab 2016;101: 871–879.
- Uhl O, Demmelmair H, Segura MT, et al: Ef- fects of obesity and gestational diabetes mel- litus on placental phospholipids. Diabetes Res Clin Pract 2015;109:364–371.
- Uhl O, Hellmuth C, Demmelmair H, et al: Dietary effects on plasma glycerophospholip- ids. J Pediatr Gastroenterol Nutr 2015;61: 367–372.
- Rzehak P, Thijs C, Standl M, et al: Variants of the FADS1 FADS2 gene cluster, blood levels of polyunsaturated fatty acids and eczema in children within the first 2 years of life. PLoS One 2010;5:e13261.
- Alsharari ZD, Riserus U, Leander K, et al: Serum fatty acids, desaturase activities and abdominal obesity – a population-based study of 60-year-old men and women. PLoS One 2017;12:e0170684.
- von Kries R, Koletzko B, Sauerwald T, et al: Breast feeding and obesity: cross sectional study. BMJ 1999;319:147–150.Arenz S, Ruckerl R, Koletzko B, von Kries R: Breast-feeding and childhood obesity – a sys- tematic review. Int J Obes Relat Metab Dis- ord 2004;28:1247–1256.
- Arenz S, Ruckerl R, Koletzko B, von Kries R: Breast-feeding and childhood obesity – a systematic review. Int J Obes Relat Metab Dis- ord 2004;28:1247–1256.
- Koletzko B, Broekaert I, Demmelmair H, et al: Protein intake in the first year of life: a risk factor for later obesity? The E.U. childhood obesity project. Adv Exp Med Biol 2005;569: 69–79.
- Koletzko B, Demmelmair H, Grote V, et al: High protein intake in young children and increased weight gain and obesity risk. Am J Clin Nutr 2016;103:303–304.
- Koletzko B, von Kries R, Closa R, et al: Lower protein in infant formula is associated with lower weight up to age 2 y: a randomized clinical trial. Am J Clin Nutr 2009;89:1836– 1845.
- Weber M, Grote V, Closa-Monasterolo R, et al: Lower protein content in infant formula reduces BMI and obesity risk at school age: follow-up of a randomized trial. Am J Clin Nutr 2014;99:1041–1051.
- Fewtrell M, Bronsky J, Campoy C, et al: Complementary feeding: a position paper by the European Society for Paediatric Gastro- enterology, Hepatology, and Nutrition (ESP- GHAN) Committee on Nutrition. J Pediatr Gastroenterol Nutr 2017;64:119–132.
- Hornell A, Lagstrom H, Lande B, Thorsdottir I: Protein intake from 0 to 18 years of age and its relation to health: a systematic literature review for the 5th Nordic Nutrition Recom- mendations. Food Nutr Res 2013;57:10.3402/ fnr.v57i0.21083.
- European-Commission: Commission Del- egated Regulation (EU) of 25.9.2015 supple- menting Regulation (EU) No 609/2013 of the European Parliament and of the Council as regards the specific compositional and infor- mation requirements for infant formula and follow-on formula and as regards require- ments on information relating to infant and young child feeding. http://ec.europa.eu/ transparency/regdoc/rep/3/2015/EN/3-2015- 6478-EN-F1-1.PDF.
- Kirchberg FF, Harder U, Weber M, et al: Di- etary protein intake affects amino acid and acylcarnitine metabolism in infants aged 6 months. J Clin Endocrinol Metab 2015;100: 149–158.
- Hellmuth C, Kirchberg FF, Lass N, et al: Ty- rosine is associated with insulin resistance in longitudinal metabolomic profiling of obese children. J Diabetes Res 2016;2016:2108909.
- Socha P, Grote V, Gruszfeld D, et al: Milk protein intake, the metabolic-endocrine re- sponse, and growth in infancy: data from a randomized clinical trial. Am J Clin Nutr 2011;94(6 suppl):1776S–1784S.
- Fleddermann M, Demmelmair H, Grote V, et al: Infant formula composition affects ener- getic efficiency for growth: the BeMIM study, a randomized controlled trial. Clin Nutr 2014;33:588–595.
- Fleddermann M, Demmelmair H, Grote V, et al: Role of selected amino acids on plasma IGF-I concentration in infants. Eur J Nutr 2017;56:613–620.
- Fleddermann M, Demmelmair H, Koletzko B: Energetic efficiency of infant formulae: a review. Ann Nutr Metab 2014;64:276–283.

