Primary Prevention of Celiac Disease: Environmental Factors with a Focus on Early Nutrition
- Celiac disease (CD) is an autoimmune disease caused by gluten, which requires a lifelong gluten-free diet.
- Breastfeeding does not prevent CD.
- The timing of gluten introduction into the infant’s diet does not influence the risk of developing CD.
- Genetic predisposition (presence of HLA-DQ2 and/or DQ8) is the main factor influencing the development of CD.
Breastfeeding is the optimal way of feeding an infant for many health-related reasons, and exclusive breastfeeding for 4–6 months of age is recommended worldwide [11, 12]. There are several mechanisms by which breastfeeding has been linked to the prevention of CD. Given that breast milk is abundant in factors involving passive immunity, such as lysozyme, lactoferrin or IgA antibodies, breastfeeding may prevent gastrointestinal infections that have been linked to CD development [13– 15]. Gut permeability, which has been shown to be decreased in breastfed infants, is another important player in CD pathogenesis [16, 17]. Additionally, breastfeeding theoretically might help with the development of tolerance to gluten by exposing an infant to some gluten fractions digested with human milk [18, 19]. Moreover, the role of the gut microbiota is also being raised in the context of CD prevention due to significant differences in microbial patterns observed in breastfed infants compared to formula-fed infants [20]. The above-listed factors may be some of the plausible explanations for the protective potential of human milk against CD.
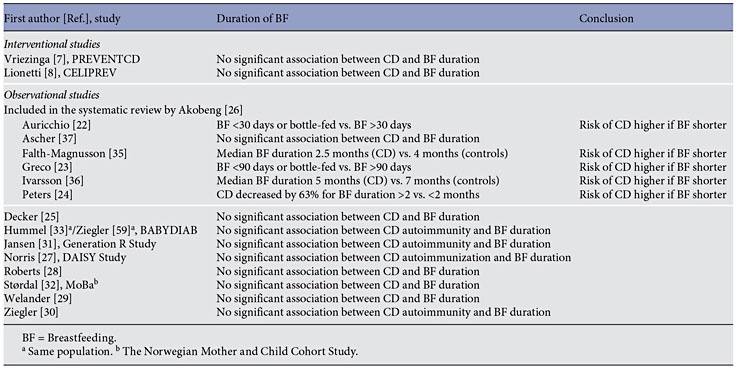
Exclusive or Any Breastfeeding and Duration of Breastfeeding
Previous studies have provided no clear evidence on whether exclusive breastfeeding compared with formula or mixed feeding reduces the risk of CD or only delays the onset of symptoms [21–24]. Contradictory results have been reported on the effect of any breastfeeding compared to no breastfeeding [24, 25]. In the systematic review by Akobeng et al. [26] (involving 6 observational studies), protection against CD with a longer duration of breastfeeding was reported. However, observational studies that followed did not confirm this finding [25, 27–30].
The most recent data from large cohort studies still brought no clear evidence with regards to the role of breastfeeding and CD prevention. A prospective Generation R cohort including 1,679 Dutch children positive for HLA-DQ2 and/or DQ8 evaluated the possible association of the timing of gluten introduction and breastfeeding duration with CD autoimmunity at 6 years of age. It was shown that breastfeeding for longer than 6 months was not related to a lower risk of CD autoimmunity [31] . Interestingly, the Norwegian Mother and Child Cohort Study (MoBa) involving more than 100,000 children showed that breastfeeding for longer than 12 months was associated with a higher risk of CD [32] . A German cohort study (BABYDIAB) observed more than 1,500 children of parents suffering from type 1 diabetes and measured islet and CD autoimmunity. No association between the duration of breastfeeding and risk of CD autoimmunity was found [33] . In the cross-sectional ETICS study (Exploring the Iceberg of Celiacs in Sweden), 2 birth cohorts were compared [34] . The duration of breastfeeding was longer in the 1997 cohort (9 months) compared to the 1993 cohort (7 months), being similar to that of the Swedish general population. The pooled results for 5 observational studies [22, 23, 25, 28, 33] showed that any breastfeeding compared with no breastfeeding had no effect on the risk of developing CD [odds ratio (OR) 0.69, 95% confidence interval (CI) 0.30–1.59] [10] . The results of breastfeeding duration on the risk of CD are summarized in table 1.
The results of both the PREVENTCD ( fig. 1 ) and CELIPREV studies showed that exclusive as well as any breastfeeding did not significantly influence the development of CD. Neither did the duration of breastfeeding ( table 1 ). Despite the above, one needs to cautiously interpret these data, as both trials were designed to evaluate the risk of CD among children who were randomly assigned to the introduction of gluten at various ages, not the effect of breastfeeding on the risk of developing CD.
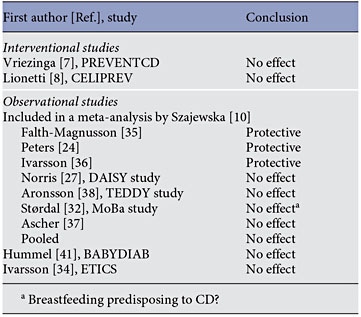
Previously, a meta-analysis by Akobeng et al. [26] suggested that breastfeeding at the time of gluten introduction might be protective. However, it was unclear whether it provided long-term prevention against CD or only delayed the onset of the disease [24, 35–37]. Results of the recently published cohort studies did not confirm the protective effect of breastfeeding at the time of gluten introduction on developing CD [31, 38]. Also, the pooled results for 7 observational studies showed that breastfeeding at the time of gluten introduction has no effect on the risk of developing CD compared with formula feeding (OR 0.88, 95% CI 0.52–1.51) [10]. The results of both recently published randomized studies, PREVENTCD and CELIPREV, did not show a protective effect of introducing gluten during breastfeeding [7, 8]. Again, caution is needed when interpreting the results, as neither of these 2 RCTs was designed to address directly the effect of breastfeeding on CD. A summary of the studies showing the effect of breastfeeding at the time of gluten introduction is presented in table 2.
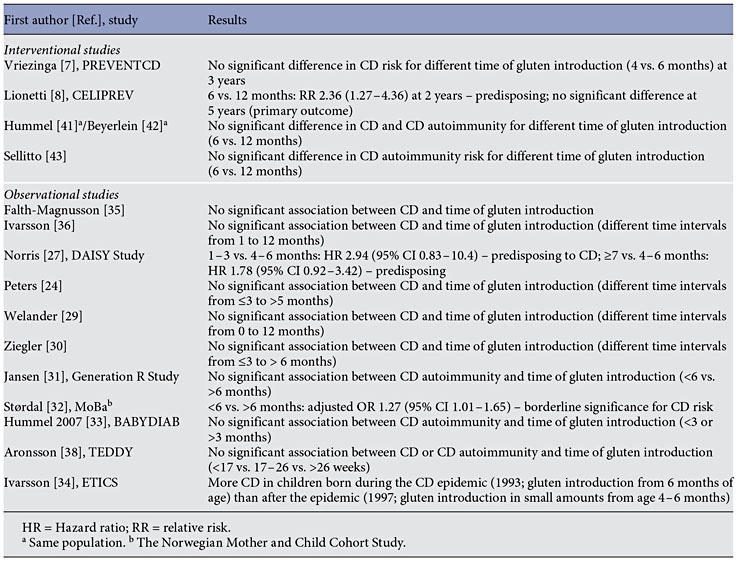
Two new large cohort studies [31, 32] reported data comparing the introduction of gluten before and after 6 months of age. The Generation R Study found that later compared to earlier exposure was not significantly associated with CD autoimmunity [31]. The Norwegian Mother and Child Cohort Study (MoBa) showed that later but not earlier exposure to gluten increased the risk of CD, although this difference was of borderline significance (adjusted OR 1.27, 95% CI 1.01–1.65) [32].
In The Environmental Determinants of Diabetes in the Young (TEDDY) study, first exposure to gluten before 17 weeks, between 17 and 26 weeks, or after 26 weeks of age was compared, and no difference was found in the risk of CD autoimmunity or CD between the groups [38] . Another cohort study (BABYDIAB) reported no difference in CD autoimmunity in infants with gluten introduction before or after 3 months of age [33].
The ETICS study of cross-sectional design compared 2 birth cohorts of 12-year-olds and found a significant difference in the total prevalence of CD in children born during the CD epidemic (in 1993, when gluten was introduced from 6 months of age) and those born after the epidemic (in 1997, with gluten introduction involving small amounts given from 4 to 6 months of age) [34].
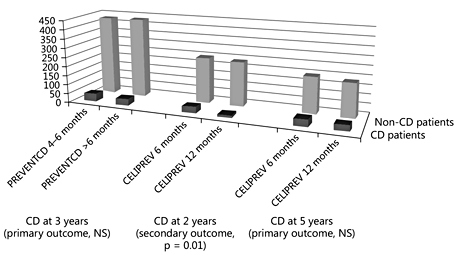
The results of interventional trials designed to assess the role of the timing of gluten introduction were published recently. In the PREVENTCD study, the risk of CD at 3 years of age was similar in the group receiving 100 mg of immunologically active gluten daily from 16 to 24 weeks of age compared to the placebo group. The cumulative incidence of CD was 5.2% (95% CI 3.6–6.8) [7]. Introduction of gluten at 6 months was compared to 12 months in 3 other studies. In the CELIPREV trial, the earlier introduction of gluten increased the risks of CD autoimmunity (16 vs. 7%, p = 0.002) and overt CD (12 vs. 5%, p = 0.01) at 2 years, but it had no effect on either risk at 5 years of age, which was the primary outcome of the study [8] . Thus, the later introduction of gluten delayed the onset of the disease without influencing the overall risk. The results for PREVENTCD and CELIPREV are presented in figure 2. Other interventional studies also reported no difference between groups in the risk of CD and/or CD autoimmunity [41– 43] (table 3).
Lastly, the risk of CD development in HLA-DQ2.5 homozygous and HLA-DQ2.2/2.5 heterozygous individuals was reported to be significantly higher than that in HLADQ2.5/ non-DQ2 heterozygous individuals. The dose of the HLA-DQ2 gene might be related to gluten epitope diversity and the risk of CD [44]. It is plausible that the amount of gluten needed for CD development is different in subjects of different genetic risk.
The PREVENTCD study reported that the amount of gluten at weaning was not related to the development of CD. This conclusion was based on the mean daily gluten intake after the intervention in a subset of participants [7]. However, of note, it was not a preplanned study outcome.
The intestinal microbiota has also been investigated as a potential pathogenetic factor in CD. Duodenal and fecal microbiota have been reported to be imbalanced in children with CD, with increased proportions of Bacteroides species and a reduction of Bifidobacterium species. A more favorable pattern was only partially restored after introducing a gluten-free diet [48, 49]. Administering Bifidobacterium together with a gluten-free diet in children with CD was investigated in a randomized placebo-controlled trial [50]. A decrease in both the numbers of the Bacteroides fragilis group and the content of secretory IgA in stools was found, which might further confirm the role of microbiota in the pathogenesis of CD. However, it still remains unclear whether the differences in microbial patterns between celiac and non-celiac individuals are a causative factor or rather a consequence of the disease. Interestingly, a possible interaction between genotype and gut microbiota predisposing to CD development has been raised in some studies and is being further explored [20]. In infants at a high genetic risk of developing CD, the bacterial pattern is significantly different from that in infants at moderate or low risk [51–53]. Further studies on the potential role of altered microbiota in infants genetically predisposed to CD might provide more insight [20].
Delivery by cesarean section and associated alterations in the development of the enteric homeostasis during the neonatal period have been suggested to influence the incidence of CD [54]. However, more recent large cohort studies have found no association between the mode of delivery and the incidence of CD [55, 56]. Vaccinations, due to modulation of immunity, have been proposed to be involved in the process of developing autoimmune diseases [57]. Scarce data available with regards to CD fail to support this hypothesis [58].
Neither the specific timing of gluten introduction nor breastfeeding protect against CD in genetically predisposed individuals
Furthermore, breastfeeding for 6 months and beyond should be promoted due to its many positive effects on health, but CD prevention should not be used as a reason to do so; there is no need to introduce gluten while the infant is still being breastfed. In regard to the mode of delivery, even though it was once suggested to be related to CD, there seems to be no support for such an association. Finally, the gut microbiota may play a greater role in the pathogenesis of CD than previously appreciated. The role of other environmental factors such as infections or vaccinations remains unclear.
- Husby S, Koletzko S, Korponay-Szabo IR, et al: Guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr 2012; 54: 136–160.
- Mustalahti, K, Catassi C, Reunanen A, et al: The prevalence of celiac disease in Europe: results of a centralized, international mass screening project. Ann Med 2010; 42: 587– 595.
- Kurppa K, Collin P, Mäki M, et al: Celiac disease and health-related quality of life. Expert Rev Gastroenterol Hepatol 2011; 5: 83–90.
- Shamir R, Hernell O, Leshno M: Cost-effectiveness analysis of screening for celiac disease in the adult population. Med Decis Making 2006; 26: 1–12.
- Hershcovici T, Leshno M, Goldin E, et al: Cost effectiveness of mass screening for celiac disease is determined by time-delay to diagnosis and quality of life on a gluten free diet. Aliment Pharmacol Ther 2010; 31: 901– 910.
- Troncone R, Ivarsson A, Szajewska H, Mearin ML; Members of European Multistakeholder Platform on CD (CDEUSSA): Review article: future research on coeliac disease – a position multistakeholder platform on celiac disease (CDEUSSA). Aliment Pharmacol Ther 2008; 27: 1030–1043.
- Vriezinga SL, Auricchio R, Bravi E, et al: Randomized feeding intervention in infants at high risk for celiac disease. N Engl J Med 2014; 371: 1304–1315.
- Lionetti E, Castellaneta S, Francavilla R, et al: Introduction of gluten, HLA status, and the risk of celiac disease in children. N Engl J Med 2014; 371: 1295–1303.
- Szajewska H, Chmielewska A, Pieścik-Lech M, et al: Systematic review: early infant feeding and coeliac disease prevention. Aliment Pharmacol Ther 2012; 36: 607–618.
- Szajewska H, Shamir R, Chmielewska A, et al: Systematic review with meta-analysis: early infant feeding and coeliac disease – update 2015. Aliment Pharmacol Ther 2015; 41: 1038–1054.
- World Health Organization: The optimal duration of exclusive breastfeeding: report of an expert consultation. Geneva, Word Health Organization, 2001. http://www.who. int/nutrition/publications/optimal_duration_ of_exc_bfeeding_report_eng.pdf (accessed September 4, 2012).
- ESPGHAN Committee on Nutrition; Agostoni C, Braegger C, Decsi T, Kolacek S, Koletzko B, Michaelsen KF, Mihatsch W, Moreno LA, Puntis J, Shamir R, Szajewska H, Turck D, van Goudoever J: Breast-feeding: a commentary by the ESPGHAN Committee on Nutrition. J Pediatr Gastroenterol Nutr 2009; 49: 112–125.
- Stene LC, Honeyman MC, Hoffenberg EJ, et al: Rotavirus infection frequency and risk of celiac disease autoimmunity in early childhood: a longitudinal study. Am J Gastroenterol 2006; 101: 2333–2340.
- Troncone R, Auricchio S: Rotavirus and celiac disease: clues to the pathogenesis and perspectives on prevention. J Pediatr Gastroenterol Nutr 2007; 44: 527–528.
- Solid LM: Breast milk against celiac disease. Gut 2002; 51: 767–768.
- Heyman M, Abed J, Lebreton C, et al: Intestinal permeability in coeliac disease: insight into mechanisms and relevance to pathogenesis. Gut 2012; 61: 1355–1364.
- Shulman RJ, Schanler RJ, Lau C, et al: Early feeding, antenatal glucocorticoids, and human milk decrease intestinal permeability in preterm infants. Pediatr Res 1998; 44: 519– 523.
- Chirdo FG, Rumbo M, Anon MC, et al: Presence of high levels of non-degraded gliadin in breast milk from healthy mothers. Scand J Gastroenterol 1998; 33: 1186–1192.
- Verhasselt V: Neonatal tolerance under breastfeeding influence. Curr Opin Immunol 2010; 22: 623–630.
- Verdu EF, Galipeau HJ, Jabri B: Novel players in coeliac disease pathogenesis: role of the gut microbiota. Nat Rev Gastroenterol Hepatol 2015; 12: 497–506.
- Nash S: Does exclusive breast-feeding reduce the risk of coeliac disease in children? Br J Community Nurs 2003; 8: 127–132.
- Auricchio S, Follo D, de Ritis G, et al: Does breast feeding protect against the development of clinical symptoms of celiac disease in children? J Pediatr Gastroenterol Nutr 1983; 2: 428–433.
- Greco L, Auricchio S, Mayer M, et al: Case control study on nutritional risk factors in celiac disease. J Pediatr Gastroenterol Nutr 1988; 7: 395–399.
- Peters U, Schneeweiss S, Trautwein EA, et al: A case-control study of the effect of infant feeding on celiac disease. Ann Nutr Metab 2001; 45: 135–142.
- Decker E, Engelmann G, Findeisen A, et al: Cesarean delivery is associated with celiac disease but not inflammatory bowel disease in children. Pediatrics 2010; 125:e1433– e1440.
- Akobeng AK, Ramanan AV, Buchan I, et al: Effect of breast feeding on risk of coeliac disease: a systematic review and meta-analysis of observational studies. Arch Dis Child 2006; 91: 39–43.
- Norris JM, Barriga K, Hoffenberg EJ, et al: Risk of celiac disease autoimmunity and timing of gluten introduction in the diet of infants at increased risk of disease. JAMA 2005; 293: 2343–2351.
- Roberts SE, Williams JG, Meddings D, et al: Perinatal risk factors and coeliac disease in children and young adults: a record linkage study. Aliment Pharmacol Ther 2009; 29: 222–231.
- Welander A, Tjernberg AR, Montgomery SM, et al: Infectious disease and risk of later celiac disease in childhood. Pediatrics 2010; 125:e530–e536.
- Ziegler AG, Schmid S, Huber D, et al: Early infant feeding and risk of developing type 1 diabetes-associated autoantibodies. JAMA 2003; 290: 1721–1728.
- Jansen MA, Tromp II, Kiefte-de Jong JC, et al: Infant feeding and anti-tissue transglutaminase antibody concentrations in the Generation R Study. Am J Clin Nutr 2014; 100: 1095–1101.
- Størdal K, White RA, Eggesbø M: Early feeding and risk of celiac disease in a prospective birth cohort. Pediatrics 2013; 132: 1202–1209.
- Hummel S, Hummel M, Banholzer J, et al: Development of autoimmunity to transglutaminase C in children of patients with type 1 diabetes: relationship to islet autoantibodies and infant feeding. Diabetologia 2007; 50: 390–394.
- Ivarsson A, Myléus A, Norström F, et al: Prevalence of childhood celiac disease and changes in infant feeding. Pediatrics 2013; 131:e687–e694.
- Falth-Magnusson K, Franzen L, Jansson G, et al: Infant feeding history shows distinct differences between Swedish celiac and reference children. Pediatr Allergy Immunol 1996; 7: 1–5.
- Ivarsson A, Hernell O, Stenlund H, et al: Breast-feeding protects against celiac disease. Am J Clin Nutr 2002; 75: 914–921.
- Ascher H, Krantz I, Rydberg L, et al: Influence of infant feeding and gluten intake on coeliac disease. Arch Dis Child 1997; 76: 113– 117.
- Aronsson CA, Lee H-S, Liu E, et al: Age at gluten introduction and risk of celiac disease. Pediatrics 2015; 135: 239–245.
- ESPGHAN Committee on Nutrition; Agostoni C, Decsi T, Fewtrell M, et al: Complementary feeding: a commentary by the ESPGHAN Committee on Nutrition. J Pediatr Gastroenterol Nutr 2008; 46: 99–110.
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA): Scientific opinion on the appropriate age for introduction of complementary feeding of infants. http://www. efsa.europa.eu/en/efsajournal/doc/1423.pdf (accessed June 9, 2015).
- Hummel S, Pfluger M, Hummel M, et al: Primary dietary intervention study to reduce the risk of islet autoimmunity in children at increased risk for type 1 diabetes: the BABYDIET study. Diabetes Care 2011; 34: 1301–1305.
- Beyerlein A, Chmiel R, Hummel S, et al: Timing of gluten introduction and islet autoimmunity in young children: updated results from the BABYDIET study. Diabetes Care 2014; 37:e194–e195.
- Sellitto M, Bai G, Serena G, et al: Proof of concept of microbiome-metabolome analysis and delayed gluten exposure on celiac disease autoimmunity in genetically at-risk infants. PLoS One 2012; 7:e33387.
- Vader W, Stepniak D, Kooy Y, et al: The HLA-DQ2 gene dose effect in celiac disease is directly related to the magnitude and breadth of gluten-specific T cell responses. Proc Natl Acad Sci USA 2003; 100: 12390– 12395.
- Kagnoff MF: Celiac disease: pathogenesis of a model immunogenetic disease. J Clin Invest 2007; 117: 41–49.
- Welander A, Tjernberg AR, Montgomery SM, et al: Infectious disease and risk of later celiac disease in childhood. Pediatrics 2010; 125:e530–e536.
- Lebwohl B, Green PHR, Murray JA: Season of birth in a nationwide cohort of coeliac disease patients. Arch Dis Child 2013; 98: 48–51.
- Collado MC, Donat E, Ribes-Koninckx C, et al: Imbalances in faecal and duodenal Bifidobacterium species composition in active and nonactive coeliac disease. BMC Microbiol 2008; 8: 232.
- Collado MC, Donat E, Ribes-Koninckx C, et al: Specific duodenal and faecal bacterial groups associated with paediatric coeliac disease. J Clin Pathol 2009; 62: 264–269.
- Olivares M, Castillejo G, Varea V, et al: Double- blind, randomised, placebo-controlled intervention trial to evaluate the effects of Bifidobacterium longum CECT 7347 in children with newly diagnosed coeliac disease. Br J Nutr 2014; 112: 30–40.
- De Palma G, Capilla A, Nadal I, et al: Interplay between human leukocyte antigen genes and the microbial colonization process of the newborn intestine. Curr Issues Mol Biol 2010; 12: 1–10.
- Sánchez E, De Palma G, Capilla A, et al: Influence of environmental and genetic factors linked to celiac disease risk on infant gut colonization by Bacteroides species. Appl Environ Microbiol 2011; 77: 5316–5323.
- De Palma G, Capilla A, Nova E, et al: Influence of milk-feeding type and genetic risk of developing coeliac disease on intestinal microbiota of infants: the PROFICEL study. PLoS One 2012; 7:e30791.
- Decker E, Engelmann G, Findeisen A, et al: Cesarean delivery is associated with celiac disease but not inflammatory bowel disease in children. Pediatrics 2010; 125:e1433– e1440.
- Emilsson L, Magnus MC, Størdal K: Perinatal risk factors for development of celiac disease in children, based on the prospective Norwegian Mother and Child Cohort Study. Clin Gastroenterol Hepatol 2015; 13: 921– 927.
- Sevelsted A, Stokholm J, Bønnelykke K, et al: Cesarean section and chronic immune disorders. Pediatrics 2015; 135:e92–e98.
- Wraith DC, Goldman M, Lambert PH: Vaccination and autoimmune disease: what is the evidence? Lancet 2003; 362: 1659–1666.
- Myléus A, Stenlund H, Hernell O, et al: Early vaccinations are not risk factors for celiac disease. Pediatrics 2012; 130:e63–e70.
- Ziegler AG, Schmid S, Huber D, et al: Early infant feeding and risk of developing type 1 diabetes-associated autoantibodies. JAMA 2003; 290: 1721–1728.