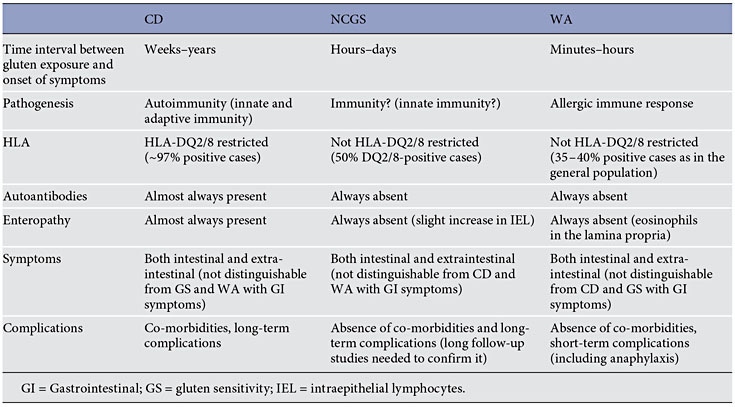
Gluten Sensitivity
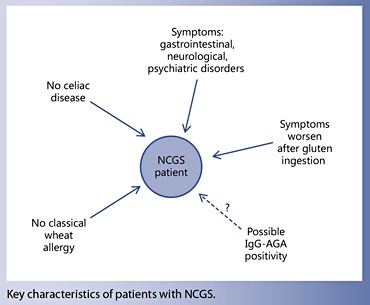
- Non-celiac gluten sensitivity (NCGS) is a syndrome characterized by intestinal and extraintestinal symptoms related to the ingestion of glutencontaining food in subjects who are not affected by either celiac disease (CD) or wheat allergy.
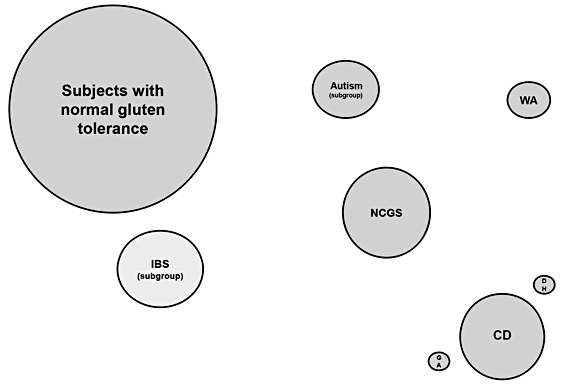
- Indirect evidence suggests that NCGS is more common than CD, the latter affecting around 1% of the general population.
- The diagnosis of NCGS is based on establishing a clear-cut cause-effect relationship between the ingestion of gluten and the appearance of symptoms by a standardized double-blind, placebo-controlled gluten challenge.
Non-celiac gluten sensitivity (NCGS) is a syndrome characterized by intestinal and extraintestinal symptoms related to the ingestion of gluten-containing food in subjects that are not affected by either celiac disease (CD) or wheat allergy (WA) [1–2]. The terminology ‘NCGS’ is still a matter of debate and could be changed into ‘non-celiac wheat sensitivity’ in the future, although this would exclude possible offending gluten-containing cereals like barley and rye. Although the first cases of NCGS were reported in the 1970s [4–5], this entity has been rediscovered recently, following the work published in 2010 by Sapone et al. [6] describing clinical and pathophysiological features of NCGS. Since then, the number of papers reporting on NCGS has grown exponentially, as well as the number of persons treated with the gluten-free diet (GFD) because of a wide array of symptoms or conditions.
The prevalence of NCGS is not clearly defined yet. In the US and UK, more than 10% of adults currently start treatment with the GFD for different reasons and duration of time; however, many of these cases are self-diagnoses not verified by a doctor [7]. Indirect evidence suggests that ‘true’ NCGS is slightly more common than CD [8], the latter affecting around 1% of the general population [9]. NCGS has been mostly described in adults, particularly in females in the age group of 30–50 years; however, pediatric case series have also been reported [10– 11].
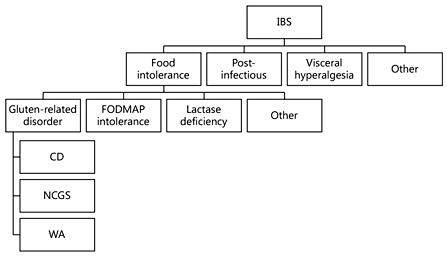
Although NCGS is triggered by the ingestion of gluten- containing cereals, the offending dietary component has not been identified yet and could include proteins that are different from gluten itself, e.g. the cereal protein amylase-trypsin inhibitors (ATIs) [3]. Evidence is also accumulating on the possible role of so-called FODMAPs (fermentable oligo-, di-, and mono-saccharides and polyols) in the induction of NCGS-like intestinal manifestations, e.g. bloating and/or diarrhea [12]. Unfortunately, NCGS and FODMAP intolerance may overlap to some extent, since gluten-rich food, particularly wheat, also contains a high amount of FODMAPs. At variance with CD, no genetic predisposing factor has been identified in NCGS patients so far. The disease mechanism/s also remains to be elucidated. Experimental data seem to indicate a possible role for an abnormal, wheat-induced innate response, as well as alterations in the small intestinal permeability (‘leaky gut’) leading to excessive absorption of gluten-derived peptides [3, 6, 13].
Clinical presentation of NCGS might be multi-systemic, and there have been a range of signs and symptoms reported in association with this condition (table 1). The latency between gluten ingestion and the appearance of symptoms is usually short, within hours or days. Since we still do not have validated biomarker(s) for the diagnosis of NCGS, the diagnostic protocol remains cumbersome and based on the evidence of a clear-cut relationship between the ingestion of gluten and clinical symptoms. Treatment of NCGS is based on the celiac-type GFD, although it is unknown whether strict avoidance of all gluten- related products is necessary or not. Since NCGS may be transient, gluten tolerance needs to be reassessed over time in patients with NCGS.
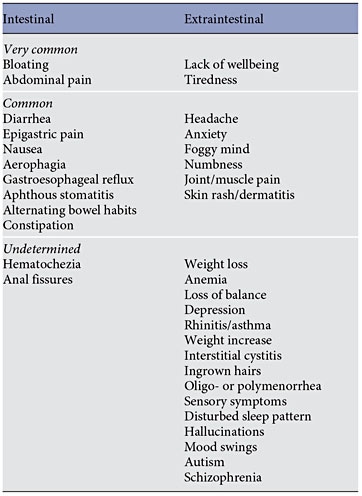
The ‘classical’ presentation of NCGS is a combination of irritable bowel syndrome (IBS)-like symptoms, including abdominal pain, bloating, bowel habit abnormalities (either diarrhea or constipation), and systemic manifestations such as ‘foggy mind’, headache, fatigue, joint and muscle pain, leg or arm numbness, dermatitis (eczema or skin rash), depression, and anemia ( table 1 ). When seen at a specialty clinic, many NCGS patients already report the causal relationship between the ingestion of gluten-containing food and worsening of symptoms. In children, NCGS manifests with IBS-like symptoms, such as abdominal pain and chronic diarrhea, while the extraintestinal manifestations seem to be less frequent (the most common extraintestinal symptom being tiredness) [6, 8, 10, 13].
In recent years, several studies have explored the relationship between the ingestion of gluten-containing food and the appearance of neurological and psychiatric disorders/symptoms like ataxia, peripheral neuropathy, schizophrenia, autism, depression, anxiety, and hallucinations (see the following paragraphs).
NCGS and IBS: A Multi-Faceted Relationship
The possibility that gluten ingestion may elicit gastrointestinal, IBS-like symptoms in non-CD patients has been confirmed by several studies [6, 8, 13, 14].
In 2011, Biesiekierski et al. [15] reported that gluten caused gastrointestinal symptoms in non-CD IBS subjects investigated by a randomized, double-blind placebo- controlled (DBPC) trial. Of 19 patients (68%) in the gluten group, 13 reported that symptoms were not adequately controlled compared with 6 of 15 (40%) on placebo. On a visual analog scale, patients were significantly worse with gluten within 1 week regarding overall symptoms, pain, bloating, satisfaction with stool consistency, and tiredness [15] . However, in a subsequent study, the same research group reached different conclusions based on the results of a different DBPC crossover trial on 37 patients with IBS/self-reported NCGS [16] . Patients were randomly assigned to a period of reduced low-fermentable, poorly absorbed, short-chain carbohydrates (FODMAPs) diet and then placed on either a gluten or whey protein challenge. In all participants, gastrointestinal complaints consistently improved during reduced FODMAP intake but significantly worsened to a similar degree when their diets included gluten or whey proteins. The FODMAP list includes fructans, galactans, fructose, and polyols that are contained in several foodstuffs, including wheat, vegetables, and milk derivatives. These results raised the possibility that the positive effect of the GFD in patients with IBS is an unspecific consequence of reducing FODMAP intake, given that wheat is one of the possible sources of FODMAPs [16] . However, it should be stressed that FODMAPs cannot be entirely and exclusively responsible for the symptoms reported by NCGS subjects, since these patients experience a resolution of symptoms while on a GFD despite continuing to ingest FODMAPs from other sources, like legumes.
Carroccio et al. [17] reviewed the clinical charts of all subjects with an IBS-like presentation who had been diagnosed with NCGS using a DBPC challenge in the years 2001–2011. Two hundred and seventy-six patients with NCGS were included. Two groups showing distinct clinical
FODMAPs cannot be entirely and exclusively responsible for the symptoms reported by NCGS subjects
characteristics were identified: NCGS alone and NCGS associated with multiple food hypersensitivity. As a group, the NCGS patients showed a higher frequency of anemia, weight loss, self-reported wheat intolerance, coexistent atopy, and food allergy in infancy than the IBS controls. There was also a higher frequency of positive serum assays for IgG/IgA anti-gliadin antibodies (AGA) and cytometric basophil activation in in vitro assay. The main histology characteristic of NCGS patients was eosinophil infiltration of the duodenal and colon mucosa. Patients with NCGS alone were characterized by clinical features very similar to those found in CD patients. Patients with multiple food sensitivity were characterized by clinical features similar to those found in allergic patients. Based on these data, authors suggested the existence of two distinct populations of subjects with NCGS, one with characteristics more similar to CD and the other with characteristics pointing towards a food allergy [17].
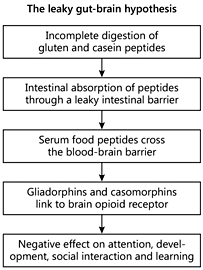
Research on the effect of diet and nutrition on autistic spectrum disorder (ASD) has been increasing in the past two decades, particularly on the symptoms of hyperactivity and attention. One of the most popular interventions for ASD is the gluten-free casein-free (GFCF) diet. The possible effect of the GFCF in children with autism is not due to underlying CD, since an association between these two conditions has never been clearly confirmed by serological screening studies [18]. It has been hypothesized that some symptoms may be caused by opioid peptides formed from the incomplete breakdown of foods containing gluten and casein. Increased intestinal permeability, also referred to as the ‘leaky gut syndrome’, has been suspected in ASD to be part of the chain of events that allows these peptides to cross the intestinal membrane, enter the bloodstream, and cross the blood-brain barrier, affecting the endogenous opiate system and neurotransmission within the nervous system (fig. 2). The resulting excess of opioids is thought to lead to behaviors noted in ASD, and the removal of these substances from the diet could determine a change in autistic behaviors [19]. The leaky gut/autism connection has fueled a strong debate within the scientific community, which is far from being settled.
A recent study has reported a high percentage of abnormal intestinal permeability test values [as established by the lactulose/mannitol (L/M) ratio] among patients with autism and their relatives compared with normal subjects. Patients with autism on a reported GFCF diet had significantly lower intestinal permeability test values compared with those who were on an unrestricted diet and controls [20]. However, Robertson et al. [21] did not detect any changes in intestinal permeability in a small cohort of ASD children. In another study, neither the L/M ratio nor behavioral scores were different between groups exposed to gluten/dairy or placebo. The changes observed were noted to be small and not clinically significant. However, this study was admittedly underpowered to show small differences [22]. The finding of IgG-class antibodies directed against food antigens is considered indirect evidence of increased intestinal permeability. Children with autism have significantly higher levels of IgG AGA (but not IgA) compared with healthy controls, particularly those with gastrointestinal symptoms [23]. Recent studies confirmed these findings and also reported an increase in antibodies directed to several other food allergens, including casein and whole milk [24].
Despite its popularity, the efficacy of the GFCF diet in improving autistic behavior remains to be proven. A 2008 Cochrane review reported that only two small randomized controlled trials investigated the effect of the GFCF diet in children with ASD (n = 35). There were only three significant treatment effects in favor of the diet intervention: overall autistic traits, mean difference (MD) = − 5.60; social isolation, MD = − 3.20, and overall ability to communicate and interact, MD = 1.70. In addition, three outcomes were not different between the treatment and control group, while differences for ten outcomes could not be analyzed because data were skewed. The review concluded that the evidence for efficacy of these diets is poor, and large-scale good-quality randomized controlled trials are needed [25]. Similar conclusions were reached by a recently published systematic review on treatment of autistic children with the GFCF diet [26].
In a two-stage randomized controlled study of the GFCF diet in children with ASD, Whiteley et al. [27] recently reported significant group improvements in core autistic and related behaviors after 8 and 12 months on diet. The results showed a less dramatic change between children having been on diet for 8 months and those on diet for 24 months, possibly reflective of a plateau effect [27]. Analyses indicated several factors to be potentially pertinent to a positive response to the dietary intervention in terms of symptom presentation. Age was found to be the strongest predictor of response, where those participants aged between 7 and 9 years seemed to derive most benefit from the dietary intervention [28]. The above data suggest that removing gluten from the diet may positively affect the clinical outcome in some children diagnosed with ASD, indicating that autism may be part of the spectrum of NCGS, at least in some cases.
On the other hand, a randomized controlled doubleblind trial was recently performed on 74 children with ASD with severe maladaptive behavior and increased urinary intestinal fatty acids binding protein (I-FABP, i.e. a marker of enterocyte damage) to test the potential ‘toxicity’ of gluten/casein. Subjects on a regular diet were randomized to receive a gluten/casein or placebo supplement for 7 days. Administrating gluten/casein to children with ASD for 1 week did not increase maladaptive behavior, gastrointestinal symptom severity or urinary I-FABP excretion [29].
In conclusion, further studies are needed to clarify the possible link between gluten ingestion and autism. Investigations are particularly required to identify phenotypes based on the best response and non-response to dietary modifications and assess any biological correlates before considering a dietary intervention.
Gluten Sensitivity and Autism
An association between schizophrenia and CD was noted in reports spanning back to the 1960s [30]. In 1986, a double-blind gluten-free/gluten-load controlled trial of 24 patients conducted by Vlissides et al. [31] showed changes in the symptom profile of schizophrenic patients in response to exclusion of gluten from the diet. On the other hand, a small blind study conducted by Potkin et al. [32] showed no differences in the clinical status of 8 schizophrenic patients on a 5-week gluten challenge in an inpatient setting, as measured by the Brief Psychiatric Rating Scale. A subsequent study by Storms et al. [33] tested 26 schizophrenic patients on a locked ward assigned to either a gluten-free or gluten-rich diet. No differences were found between the groups on their performance in a battery of psychological tests. A recent study using blood samples from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) found that 5.5% of the subjects with schizophrenia had a high level of anti-tTG antibodies (compared to 1.1% in the healthy control sample) and 23.1% had AGA IgG positivity compared with 3.1% of controls. Interestingly enough, a large proportion of transglutaminase (tTG)-positive subjects were endomysial antibody (EMA) negative, questioning the possibility that their tTG positivity was related to CD. Indeed, only 2% of schizophrenic patients fulfilled the CD diagnostic criteria (both anti-tTG and EMA positive), questioning the role of CD in schizophrenia [34]. Additional studies revealed that most of the tTG-positive subjects were tTG-6 positive, suggesting that these antibodies are more a biomarker of neuroinflammation than CD [35]. This study indicated the existence of a specific immune response to gluten in some of these patients, probably related to NCGS. Other studies confirmed the high prevalence of AGA among people with schizophrenia [36]; however, the exact mechanism underlying the observed improvement of symptoms with the GFD in some patients has remained elusive.
In 2014, Genuis and Lobo [37] reported on a patient who, around the age of 4 or 5 years, began to experience recurrent gastrointestinal problems, as well as the onset of frequent visual and auditory hallucinations. These experiences occurred on a nearly daily basis and were ‘just a part of (her) life’. As she grew older, she also found these hallucinations quite distracting, rendering her unable to concentrate adequately in school or to study for exams. The hallucinations as well as the gastrointestinal symptoms continued throughout her childhood and teenage years, causing her to miss considerable amounts of school. After attending nutrition lectures, the patient was introduced to the idea of gluten sensitivity and decided to abstain from gluten exposure. After eliminating gluten, her gastrointestinal symptoms and hallucinations completely abated, and she felt an improvement in her ability to concentrate. When reexposed to gluten, relapse consistently occurred within 3–5 h and would result in significant disorientation and departure from reality. At the follow-up visit (more than 12 months of treatment with the GFD), she had no further exposure to gluten and was symptom free with no intestinal or neuropsychiatric complaints [37].
A similar case report has been recently published by our group. We described the clinical history of a 14-yearold girl seen at our outpatient gastroenterology clinic because of psychotic symptoms that were apparently associated with gluten consumption. Two years before, she had become increasingly irritable and started to report daily headache and concentration difficulties. Her symptoms worsened presenting with severe headache, sleeping problems, and behavior alterations, with several unmotivated crying spells and apathy. Psychiatric symptoms worsened, and she began to have complex hallucinations. These involved vivid scenes either with family members (she heard her sister and her boyfriend having bad discussions) or without (she saw people coming out of the television to follow and scare her), and hypnagogic hallucinations when she relaxed in bed. An accurate neurological and general workup yielded a normal result.
Treatment with olanzapine was started, but the psychotic symptoms persisted. In November 2013, due to gastrointestinal symptoms and weight loss, a nutritionist was consulted and a GFD was recommended for symptomatic treatment; unexpectedly, within 1 week of GFD, not only the intestinal but also the psychiatric symptoms improved dramatically. After some months, a DBPC gluten challenge definitely confirmed the diagnosis of NCGS. During the second day of the wheat flour challenge, the girl presented headache, halitosis, abdominal distension, mood disorders, fatigue, and poor concentration, and three episodes of severe hallucinations (that were videorecorded). The girl refused to continue the gluten challenge and went back to the GFD, with a complete and persistent regression of all symptoms within 1 week. Our case report confirms the existence of ‘gluten psychosis’ as a possible manifestation of NCGS [38].
The diagnosis of NCGS is based on establishing a clear-cut cause-effect relationship between the ingestion of gluten and the appearance of symptoms
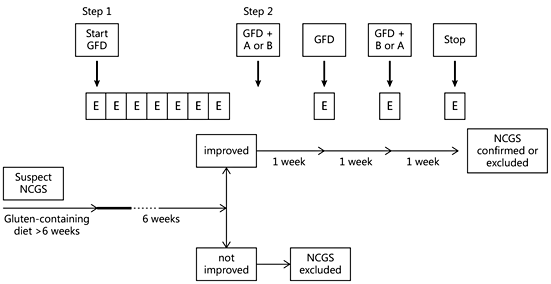
In both step 1 and step 2, the clinical evaluation is performed using a self-administered instrument incorporating a modified version of the Gastrointestinal Symptom Rating Scale (GSRS) [40] . The instrument also includes items evaluating the extraintestinal NCGS manifestations. The patient identifies one to three main symptoms that will be quantitatively assessed using a numerical rating scale with a score ranging from 1 (mild) to 10 (severe).
Step 1: Definition of a Patient Responsive to the GFD (Patient on a Gluten-Containing Diet)
The following actions establish responsiveness to the GFD. (1) A patient on a gluten-containing diet is assessed by the diagnostic questionnaire at weeks –2, –1, and 0 to establish baseline symptoms. (2) At time 0, the GFD is started after detailed explanation (preferably by a dietitian). (3) Timeline: at least 6 weeks of verified GFD. Although the amelioration of symptoms is expected shortly after starting the GFD, a prolonged observation is needed to properly investigate the causal relationship, particularly for fluctuating symptoms (e.g. headache). (4) Data recording: weekly completion of the questionnaire from week 0 to 6. The patient will identify one to three main symptoms. The response parameters are those with an initial score of at least 3 on the numerical rating scale.
The response is assessed for each parameter separately. A symptomatic response is a decrease of at least 30% of the baseline score. Responders are defined as patients who fulfill the response criteria (>30% reduction of one to three main symptoms or at least one symptom with no worsening of others) for at least 50% of the observation time (i.e. at least three of six weekly evaluations). The diagnosis of NCGS is excluded in subjects failing to show a symptomatic improvement after 6 weeks of GFD. GFD-unresponsive patients should be investigated for other possible causes of symptoms, e.g. intolerance to FODMAPs or small bowel bacterial overgrowth.
Step 2: The Gluten Challenge (Patient on the GFD)
In view of the currently high rate of perceived gluten sensitivity and the possible placebo effect of any dietary intervention, the DBPC gluten challenge demonstration of a clear-cut relationship between gluten ingestion and symptoms appearance is a crucial step in the diagnostic algorithm of NCGS. The gluten challenge includes a 1-week challenge followed by a 1-week washout of strict GFD and by the crossover to the second 1-week challenge. The duration of the challenge period may occasionally be longer than 1 week in patients showing fluctuating symptoms, such as headache or neurobehavioral problems. The questionnaire is self-administered and filled in at baseline and daily during the first 7-day challenge (or less if symptoms prevent completion of 7 days), the washout period, and the second 7-day challenge (or less if symptoms prevent completion of 7 days). During the challenge, the patient will identify and report one to three main symptoms, without necessarily filling in the full questionnaire. A variation of at least 30% between the gluten and the placebo challenge should be detected to discriminate a positive from a negative result.
The suggested dose of gluten to be used for the challenge is 8 g, which is both close to the average daily intake of gluten in Western countries (10–15 g) [41] and easy to mix with the vehicle. As far as the gluten ‘vehicle’ is concerned, gelatin capsules are discouraged. The best-suited vehicle is yet to be developed, for instance it could take the form of a muesli bar, bread, or muffin, possibly different in children and adults. The vehicle should contain cooked, homogeneously distributed gluten and should be analyzed in order to know exactly the content of the pro-inflammatory factor ATIs. The gluten preparations should be prepared/tested for ATI bioactivity to contain at least 0.3 g of ATIs/8 g of gluten or gluten should be used with a defined ATI content. The vehicle should be FODMAP free. The placebo vehicle must be completely gluten-free. Gluten and placebo preparations must be undistinguishable in look, texture, and taste as well as balanced in fibers, carbohydrate, fat, and possibly protein content.
The threshold of a 30% increment in symptoms is somewhat arbitrary and needs scientific validation. Patients showing a negative gluten challenge should be investigated for other possible causes of IBS-like symptoms, e.g. intolerance to FODMAPs or small bowel bacterial overgrowth. The flow diagram suggested for the confirmation of a NCGS diagnosis is shown in figure 3.
Although the most specific CD serological markers, such as IgA-class anti-tTG and EMA, are negative in NCGS patients by definition, IgG-class AGA directed against native gliadin are found more frequently in these cases (about 50%) than in the general population, when eating a gluten-containing diet. Therefore, the finding of isolated IgG-AGA positivity may be a clue to the diagnosis of NCGS. When initially positive, IgG AGA normalize more quickly in NCGS than CD patients after starting treatment with the GFD [42]. However, the determination of IgG AGA cannot be recommended for clinical use due to the poor specificity of this test.
- Sapone A, Bai JC, Ciacci C, Dolinsek J, Green PH, Hadjivassiliou M, Kaukinen K, Rostami K, Sanders DS, Schumann M, et al: Spectrum of gluten-related disorders: consensus on new nomenclature and classification. BMC Med 2012; 10: 13.
- Catassi C, Bai JC, Bonaz B, Bouma G, Calabrò A, Carroccio A, Castillejo G, Ciacci C, Cristofori F, Dolinsek J, et al: Non-celiac gluten sensitivity: the new frontier of gluten related disorders. Nutrients 2013; 5: 3839–3853.
- Schuppan D, Zevallos V: Wheat amylase trypsin inhibitors as nutritional activators of innate immunity. Dig Dis 2015; 33: 260–263.
- Ellis A, Linaker BD: Non coeliac gluten sensitivity? Lancet 1978; 1: 1358–1359.
- Cooper BT, Holmes GK, Ferguson R, Thompson RA, Allan RN, Cooke WT: Gluten-sensitive diarrhea without evidence of celiac disease. Gastroenterology 1981; 81: 192–194.
- Sapone A, Lammers K.M, Mazzarella G, Mikhailenko I, Cartenì M, Casolaro V, Fasano A: Differential mucosal IL-17 expression in two gliadin-induced disorders: gluten sensitivity and the autoimmune enteropathy celiac disease. Int Arch Allergy Immunol 2010; 152: 75–80.
- Aziz I, Lewis NR, Hadjivassiliou M, Winfield SN, Rugg N, Kelsall A, Newrick L, Sanders DS: A UK study assessing the population prevalence of self-reported gluten sensitivity and referral characteristics to secondary care. Eur J Gastroenterol Hepatol 2014; 26: 33–39.
- Volta U, Bardella MT, Calabrò A, Troncone R, Corazza GR; Study Group for Non-Celiac Gluten Sensitivity: An Italian prospective multicenter survey on patients suspected of having non-celiac gluten sensitivity. BMC Med 2014; 12: 85.
- Lionetti E, Gatti S, Pulvirenti A, Catassi C: Celiac disease from a global perspective. Best Pract Res Clin Gastroenterol 2015; 29: 365–379.
- Francavilla R, Cristofori F, Castellaneta S, Polloni C, Albano V, Dellatte S, Indrio F, Cavallo L, Catassi C: Clinical, serologic, and histologic features of gluten sensitivity in children. J Pediatr 2014; 164: 463–467.
- Feldman MF, Bird JA: Clinical, serologic, and histologic features of gluten sensitivity in children. Pediatrics 2014; 134(suppl 3): S157–S158.
- Gibson PR, Varney J, Malakar S, Muir JG: Food components and irritable bowel syndrome. Gastroenterology 2015; 148: 1158–1174.
- Vazquez-Roque MI, Camilleri M, Smirk T, Murray JA, Marietta E, O’Neill J, Carlson P, Lamsam J: A controlled trial of gluten-free diet in patients with irritable bowel syndrome-diarrhea: effects on bowel frequency and intestinal function. Gastroenterology 2013; 144: 903–911.
- Di Sabatino A, Volta U, Salvatore C, Biancheri P, Caio G, De Giorgio R, Di Stefano M, Corazza GR: Small amounts of gluten in subjects with suspected nonceliac gluten sensitivity: a randomized, double-blind, placebocontrolled, cross-over trial. Clin Gastroenterol Hepatol 2015; 13: 1604–1612.e3.
- Biesiekierski JR, Newnham ED, Irving PM, Barrett JS, Haines M, Doecke JD, Shepherd SJ, Muir JG, Gibson PR: Gluten causes gastrointestinal symptoms in subjects without celiac disease: a double-blind randomized placebo-controlled trial. Am J Gastroenterol 2011; 106: 508–514.
- Biesiekirski JR, Peters SL, Newnham ED, Rosella O, Muir JG, Gibson PR: No effects of gluten in patients with self-reported nonceliac gluten sensitivity following dietary reduction of low-fermentable, poorly absorbed, short-chain carbohydrates. Gastroenterology 2013; 145, 320–328.
- Carroccio A, Mansueto P, Iacono G, Soresi M, D’Alcamo A, Cavataio F, Brusca I, Florena AM, Ambrosiano G, Seidita A, Pirrone G, Rini GB: Non-celiac wheat sensitivity diagnosed by double-blind placebo-controlled challenge: exploring a new clinical entity. Am J Gastroenterol 2012; 107: 1898–1906.
- Batista IC, Gandolfi L, Nobrega YK, Almeida RC, Almeida LM, Campos Junior D, Pratesi R: Autism spectrum disorder and celiac disease: no evidence for a link. Arq Neuropsiquiatr 2012; 70: 28–33.
- Marcason W: What is the current status of research concerning use of a gluten-free, casein-free diet for children diagnosed with autism? J Am Diet Assoc 2009; 109: 572.
- De Magistris L, Familiari V, Pascotto A, Sapone A, Frolli A, Iardino P, Carteni M, de Rosa M, Francavilla R, Riegler G, et al: Alterations of the intestinal barrier in patients with autism spectrum disorders and in their first-degree relatives. J Pediatr Gastroenterol Nutr 2010; 51: 418–424.
- Robertson MA, Sigalet DL, Holst JJ. Meddings JB, Wood J, Sharkey KA: Intestinal permeability and glucagon-like peptide-2 in children with autism: a controlled pilot study. J Autism Dev Disord 2008; 38: 1066–1071.
- Navarro F, Pearson DA, Fatheree N, Mansour R, Hashmi SS, Rhoads JM: Are ‘leaky gut’ and behavior associated with gluten and dairy containing diet in children with autism spectrum disorders? Nutr Neurosci 2015; 18: 177–185.
- Lau NM, Green PH, Taylor AK, Hellberg D, Ajamian M, Tan CZ, Kosofsky BE, Higgins JJ, Rajadhyaksha AM, Alaedini A: Markers of celiac disease and gluten sensitivity in children with autism. PLoS One 2013; 8:e66155.
- De Magistris L, Picardi A, Siniscalco D, Riccio MP, Sapone A, Cariello R: Antibodies against food antigens in patients with autistic spectrum disorders. Biomed Res Int 2013; 2013: 729349.
- Millward C, Ferriter M, Calver S, Connell-Jones G: Gluten- and casein-free diets for autistic spectrum disorder. Cochrane Database Syst Rev 2008; 2:CD003498.
- Marí-Bauset S, Zazpe I, Mari-Sanchis A, Llopis-González A, Morales-Suárez-Varela M: Evidence of the gluten-free and casein-free diet in autism spectrum disorders: a systematic review. J Child Neurol 2014; 29: 1718–1727.
- Whiteley P, Haracopos D, Knivsberg AM, Reichelt KL, Parlar S, Jacobsen J, Seim A, Pedersen L, Schondel M, Shattock P: The ScanBrit randomised, controlled, singleblind study of a gluten- and casein-free dietary intervention for children with autism spectrum disorders. Nutr Neurosci 2010; 13: 87–100.
- Pedersen L, Parlar S, Kvist K, Whiteley P, Shattock P: Data mining the ScanBrit study of a gluten- and casein-free dietary intervention for children with autism spectrum disorders: behavioural and psychometric measures of dietary response. Nutr Neurosci 2014; 17: 207–213.
- Pusponegoro HD, Ismael S, Firmansyah A, Sastroasmoro S, Vandenplas Y: Gluten and casein supplementation does not increase symptoms in children with autism spectrum disorder. Acta Paediatr 2015, Epub ahead of print.
- Dohan FC: Cereals and schizophrenia data and hypothesis. Acta Psychiatr Scand 1966; 42: 125–152.
- Vlissides DM, Venulet A, Jenner FA: A double-blind gluten-free/gluten-load controlled trial in a secure ward population. Br J Psychiatry 1986; 148: 447–452.
- Potkin SG, Weinberger D, Kleinman J, Potkin SG, Weinberger D, Kleinman J, Nasrallah H, Luchins D, Bigelow L, Linnoila M, et al: Wheat gluten challenge in schizophrenic patients. Am J Psychiatry 1981; 138: 1208–1211.
- Storms LH, Clopton JM, Wright C: Effects of gluten in schizophrenics. Arch Gen Psychiatry 1982; 39: 323–327.
- Cascella NG, Kryszak D, Bhatti B, Gregory P, Kelly DL, Mc Evoy JP, Fasano A, Eaton WW: Prevalence of celiac disease and gluten sensitivity in the United States clinical antipsychotic trials of intervention effectiveness study population. Schizophr Bull 2011; 37: 94–100.
- Cascella NG, Santora D, Gregory P, Kelly DL, Fasano A, Eaton WW: Increased prevalence of transglutaminase 6 antibodies in sera from schizophrenia patients. Schizophr Bull 2013; 39: 867–871.
- Dickerson F, Stallings C, Origoni A, Vaughan C, Khushalani S, Leister F, Yang S, Krivogorsky B, Alaedini A, Yolken R: Markers of gluten sensitivity and celiac disease in recent-onset psychosis and multi-episode schizophrenia. Biol Psychiatry 2010; 68: 100–104.
- Genuis SJ, Lobo RA: Gluten sensitivity presenting as a neuropsychiatric disorder. Gastroenterol Res Pract 2014; 2014: 293206.
- Lionetti E, Leonardi S, Franzonello C, Mancardi M, Ruggieri M, Catassi C: Gluten psychosis: confirmation of a new clinical entity. Nutrients 2015; 7: 5532–5539.
- Catassi C, Elli L, Bonaz B, et al: Diagnosis of non-celiac gluten sensitivity (NCGS): The Salerno Experts’ Criteria. Nutrients 2015; 7: 4966–4977.
- Kulich KR, Madisch A, Pacini F, Piqué JM, Regula J, van Rensburg CJ, Ujszászy L, Carlsson J, Halling K, Wiklund IK: Reliability and validity of the Gastrointestinal Symptom Rating Scale (GSRS) and Quality of Life in Reflux and Dyspepsia (QOLRAD) questionnaire in dyspepsia: a six-country study. Health Qual Life Outcomes 2008; 6: 12.
- Van Overbeek FM, Uil-Dieterman IG, Mol IW, Köhler-Brands L, Heymans HS, Mulder CJ: The daily gluten intake in relatives of patients with coeliac disease compared with that of the general Dutch population. Eur J Gastroenterol Hepatol 1997; 9: 1097–1099.
- Caio G, Volta U, Tovoli F, De Giorgio R: Effect of gluten free diet on immune response to gliadin in patients with non-celiac gluten sensitivity. BMC Gastroenterol 2014; 14: 26.