What is the evidence that nutrition affects brain outcomes in preterm infants?
What is the evidence that nutrition affects brain outcomes in preterm infants?
Nicholas D.Embleton
The last 30 years have seen a dramatic in crease in survival of infants born preterm. The reasons for these improvements are complex, but there is strong reason to believe that improved attention to nutritional management has had a key impact on improving shortand longterm outcomes.
In turn, improvements in survival have meant that we are caring for increasingly fragile infants, some of whom have complex nutritional requirements. Despite advances in understanding and practice, adverse cognitive outcome remains the most serious consequence in preterm infants who survive.
The energy requirements of the brain are very high. To illustrate this, take this example from Geraint Thomas, who won the Tour de France in 2018. This athlete cycles up and down mountains for 3 wk and during this time he consumes 7,000 kcal/day, which is equivalent to 100 kcal/kg/day. In contrast, our smallest babies in the NICU require at least 120–130 kcal/kg/day. Most of this energy expenditure hap- pens in the brain. So it is very easy to understand how even a short-term energy deficit is associated with a high risk of poor brain development.
Brain development is particularly rapid in the 3rd trimester and infancy: between 24 wk gestation and 2 years of age, human infants acquire around 90% of their brain size. During this period, a series of neuro-anatomical developmental processes must take place in a time-coordinated fashion if complex behavioural, cognitive and motor circuits are to develop normally. Normal brain development may be prevented due to damage associated with hypoxic or ischaemic events, brain haemorrhage, or ‘cytokine’ damage associated with systemic sepsis syndromes. Nutritional management – feeding rates, breast milk, etc. – may impact on the incidence of sepsis and necrotising enterocolitis (NEC) and thus, in turn, affect brain outcomes (Chart 1).
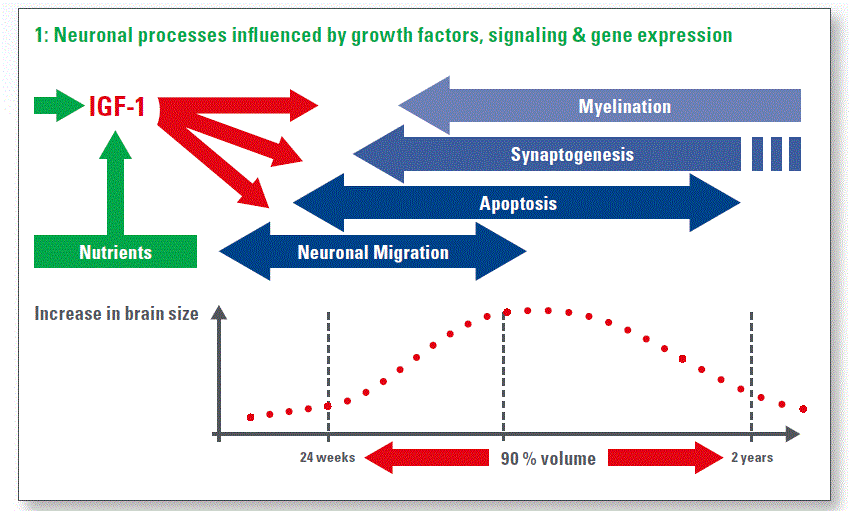
When you put together the complex neurological process, the complexity of the NICU environment and the fact that we can see a sub-optimal nutritional status, it is perhaps not surprising that we see very large numbers of preterm babies with abnormal neurocognitive outcome, neurocognitive phenotype.
At least five different interrelated mechanisms associate nutrition with brain growth. We need:
- Nutrients for tissue substrate: macro- and micronutrients
- Energy to drive the system: carbohydrates, lipids, proteins
- Signaling and growth factors: energy intake, branched-chain amino acids, IGF-1, etc.
- Impacts on gene expression: folate, B12, iron, choline, etc.
- Prevention of disease: breast milk reduces NEC and sepsis and everything that reduces NEC and sepsis improves brain growth.
Why is malnutrition so common? I think it is the complex situation in the NICU. The focus is what we can see and measure – and we measure a lot. But malnutrition is invisible and there is no machine or monitor that sounds an alarm when nutrient intakes are inadequate. The impact of nutrient deficiency – or excess – on preterm brain depends on the amount and type of nutrient, but also on the timing and duration of the exposure.
In the first few days and weeks, parenteral nutrition (PN) is the only reliable means of delivering adequate macronutrients to very preterm infants. Failure to provide adequate macronutrients in just the first week is associated with worse developmental outcome in infancy; but despite the strong observational evidence, there are no controlled trials of PN that clearly show improvements in brain outcomes.
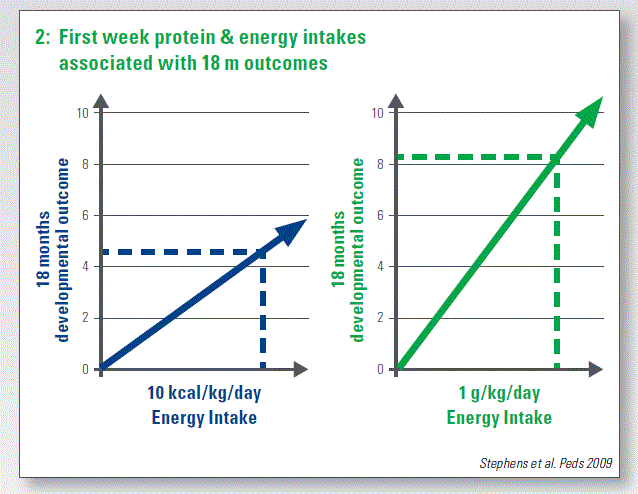
A study shows 1 wk protein and energy intakes associated with 18 mo outcomes (n = 124; £ 1,000 g; mean 25 wk gestation). For 10 kcal/kg/day energy intake, you have 4.6 advantage in the MDI Bayley at 18 mo and an advantage of 8.2 for 1 g/kg/day protein intake (Stephens at al. Peds 2009) (Chart 2).
2 RCTs in the UK show different results: in SCAMP (Morgan et al. 2015), there was better head growth; conversely, in NEON (Uthaya et al. 2016) there was worse head growth with higher AA intakes. However, head size is not a functional outcome. We looked into 10 RCTs on PN and higher amounts of amino acids (AA) between 2007–2017 (Embleton et al. 2018). Basically only 2 RCTs show any effect of AA on brain outcomes. The conclusion is: there is no consistent evidence of brain benefit from higher AA intakes or more rapid increases.
In summary: RCT evidence is limited, optimal PN macronutrient regime is uncertain, there is no evidence that taking 2–3 days to achieve recommended PN intakes is harmful, and only few RCTs explored energy intakes.
Enteral macronutrient intakes
RCTs on enteral macronutrient intakes and brain outcomes with long-term follow-up showed unequivocal evidence that higher macronutrient intakes result in better brain outcomes. But these studies are quite old, the infant populations are different now, and the studies were not able to define ‘optimal’ intake (e.g. 3.5 g or 4 g or 4.5 g protein intakes). Other nutrition intakes might affect the brain in high-risk infants.
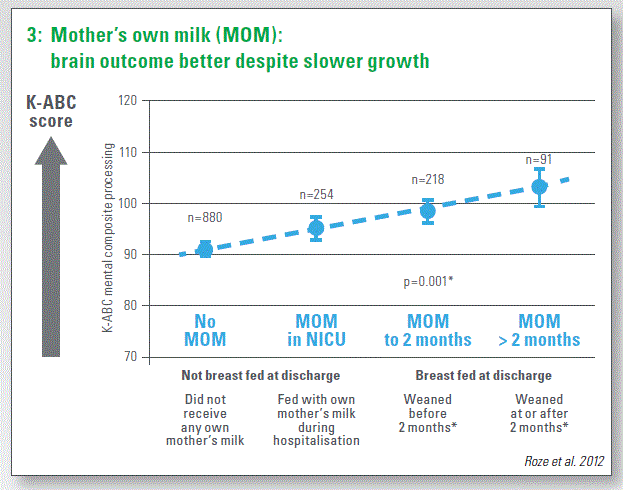
Mother’s own milk (MOM):
The strongest data linking nutrition in preterm infants and later brain outcomes support the use of mother’s own breast milk. This tends to show a dose-response effect on a range of improved brain outcomes (Chart 3).
Most mothers unfortunately do not provide 100% of their babies’ requirements, so it is important to determine how best to improve MOM supply. Is donor milk beneficial and does fortifier improve brain volume?
Donor human milk (DHM):
A study in Canada (n= 363; Mean birth weight 996g; 27 wk gestation) compared the effect of supplemental DHM with formula on neurodevelopment. There was no benefit of DHM on brain outcome.
Nutrient fortification of human milk:
There are 14 trials (n= 1071) showing small increases in weight and/or length. None of the studies include any benefit for long-term outcomes. And the RCTs are small and methodologically weak.
Specific nutritional interventions:
There are also trials exploring specific nutrients (e.g. DHA or iodine), but there are few data to show that providing micronutrients in excess of recommended levels is beneficial, although most studies are under-powered for cognitive outcomes.
Postdischarge
More recently, studies have focused on the post-discharge period. These show that whilst higher macronutrient intakes may result in greater growth rates and have positive effects on body composition, there are no strong data to show that enriched milk formula or fortification of breast milk results in improved brain outcomes in the longer term.
Observational data point to the importance of breast milk even in the post-discharge period, despite the slower weight gain that is often observed. Studies have also begun to explore the role of in- creased levels of specific nutrients such as choline, with one recent trial suggesting that this may improve developmental outcomes in preterm and other infants at high risk of brain injury (Caudill et al. FASEB journal 2018).
Conclusions
- Focus on mother’s own milk (MOM)
- Deficient macronutrient intakes remain common
- Optimal intakes remain uncertain
- Macronutrient enrichment postdischarge in highrisk
- Ensure micronutrients are not deficient
- Iron, zinc, iodine, essential lipids, etc.
- ‘Nutritional’ prevention of NEC or sepsis
- Urgent need for welldesigned trials
- Multiple areas of future promise
