Modifying the Balance – What is the Role of the Gut Microbiome in Allergic Disease?
Modifying the Balance – What is the Role of the Gut Microbiome in Allergic Disease?
Christina E. West
The immune system is a system of cells and tissues that protects us against invading pathogens. It must learn how to provide tolerance to “non threats” such as food components, commensal microbiota and to the organism itself. So it must actually learn how to distinguish “harmless” from “dangerous” – and this is an active process.
Immune System Development
Immune cells and organs proliferate rapidly in the first trimester and the development of secondary lymphoid organs is nearly complete at birth. However, quite recent work indicates that these organs, particularly the GALT, remain highly responsive to environmental stimuli (antigens and allergens) throughout life.
Declining biodiversity and aberrant intestinal colonization is a main theme in the “allergy epidemic.” Complex microbial communities in the gastrointestinal tract have a symbiotic relationship with the host,and microbial exposures in the perinatal period seem to be critical for the ontogeny of both innate and adaptive immunity.
Colonization of the mucosal surfaces appears to be vital for the establishment of tolerance. Delay or disruption in the colonization of the gastrointestinal tract may result in dysregulated immune responses. This has been clearly shown in animal models and there is also indirect evidence in humans. For example, delivery by cesarean section is associated with disturbed intestinal colonization patterns and increased risk of both allergic and autoimmune disease. But we do not yet know exactly how the mechanism of tolerance establishes itself in humans.
Gut microbiota have also been demonstrated to enhance the integrity and functions of the gut barrier. Gut microbiota will increase secretory IgA production locally and also induce the goblet cells to produce mucin, thereby enhancing the gut barrier. The gut microbiota can also be involved in crosstalk with distant organs as bacterial ligands, bacterial metabolites and immune cells may enter the systemic circulation and seed distant organs e.g. the lung.
Toll-like receptors (TLR) are believed to be ancient gatekeepers in innate immunity; they are expressed on a number of epithelial and endothelial cells, leukocyte subsets in blood. TLR activation can either increase or decrease the suppressor activity of Tregulatory cells, thus providing an important link between innate and adaptive immunity.
In a study within a high-risk cohort in Perth, where blood was first sampled at birth and afterwards during the first five years of life, infants that later developed allergic disease showed increased inflammatory responses following TLR activation in early life compared with healthy children. However, these responses failed to develop into mature Th1 type responses in allergic children (Tulic MK et al., JACI 2011).
Sterile womb paradigm versus in utero colonization hypothesis
The sterile womb paradigm asserts that the fetus and the placenta are sterile and that gut microbiota is acquired after birth. Vaginal delivery will obviously have a huge influence of this process.
The emerging in utero colonization hypothesis posits that the placenta harbors its own microbiota and that the colonization of the gut begins already in utero. More research is needed to confirm the latter hypothesis.
Building on the results from the high-risk cohort in Perth, where babies who went on to develop allergic disease had exaggerated innate immune responses (Tulic MK et al., JACI 2011), we hypothesized that this immune dysfunction might be dependent on microbial stimuli very early in life. So in another high-risk cohort, in which atopic mothers were included, we sampled feces from the mothers in the 3rd trimester and then afterwards from their infants at 1 week, 1 mo and 12 mo of age. Blood was drawn at 6 mo of age and PBMC were activated with microbial ligands. We studied microbial development in babies that had eczema, plus evidence of sensitization or had food allergy. We paired the despite their heredity, were non-sensitized and had no eczema (Chart 1).
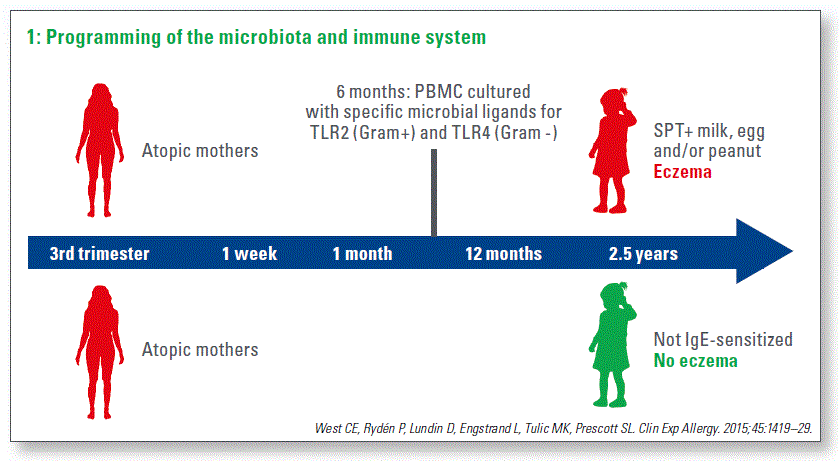
There were both maternal effects and also postnatal effects. In pregnancy, there was reduced diversity microbial development to healthy controls, which of the main phylum Bacteridetes in mothers whose infants developed IgE-associated eczema. Although this was not seen in their infants, it could be consistent with results from a Swedish study that found reduced Bacteroides in infants who developed IgE-associated eczema (Abrahamsson TR et al., JACI 2012). In our study, we found reduced Ruminococcaceae in infants that developed IgE-associated eczema. Notably, the underrepresentation of Ruminococcus in these infants was associated with exagerrated innate inflammatory immune responses (West CE, et al. Clin Exp Allergy 2015). Interestingly, underrepresentation of Ruminococcaceae was also a feature in food sensitized 1-year-olds in a Canadian cohort (Azad MB et al., Clin Exp Allergy 2015).
Very recently, we studied the longitudinal gut microbial development in allergic disease in more detail as we had collected stool samples in a probiotic allergy prevention trial in infancy. We did a follow up at 8 years of age including a very thorough clinical assessment and collection of biological specimens (West CE et al., Allergy 2013). We clinically phenotyped the children at 8 years of age and found 21 children which had IgE-associated allergic disease. Seventy-two children served as healthy controls as they were neither IgE sensitized nor did they show any other allergic manifestations.
Because we could study these children longitudinally from infancy to school age, we could identify both temporal and consistent underrepresentation of specific taxa in allergic diseases (Sjödin KS et al., Allergy in press). Ruminoccous was transiently underrepresented whereas Prevotella, Coprococ- cus and Bacteroides were consistenly underrepresented. Another novel finding is that Faecalibacterium, which is suggested as a bacterial biomarker for gut health, correlated with the T cell regulatory markers IL10 and FOXP3 in allergic children at 8 years of age. In addition, we found transient and minor effects of probiotic intake so, during the intervention, there was a higher abundance of Lactobacillus at 6 mo of age; when we looked at 8 years of age there were no effects on the global microbiota nor were there any long-lasting effects on the Lacto- bacillus population.
Importance of Bacteroidetes
One of the functions of Bacteroidetes is that they stimulate epithelial mucin production and, together with Prevotella, Coprococcus and Bacteroides, they have the capacity to ferment carbohydrates to produce butyrate, with both nutritive and an- ti-inflammatory effects. Very recently, Bacteroides-derived fecal sphingolipids were negatively associated with food allergy (Lee-Sarwar K et al., JACI 2018).
In summary, this is the first study to have actually looked at the longitudinal development of gut microbiota in relation to IgE associated diseases (Sjödin KS et al., Allergy in press). Although there was a temporal underrepresentation of Ruminococcus, one important finding is that it was actually consistent underrepresentation of Bacteroides, Prevotella and Coprococcus.
The dysbiosis seen in a number of inflammatory non-communicable diseases has aroused huge interest and prompted researchers to use various microbiota modulation techniques to try to favorably modulate gut microbiota to improve immunological and metabolic outcomes and also influence the gut- brain axis. We do have to keep in mind that nutrition and gut microbiota are interrelated, so there are a number of dietary exposures that can have effects both on immunological programming and the gut microbiota. These include pre- and probiotics, dietary fibers, folate and antioxidants, when and how we introduce allergenic foods and n3-PUFA. So the aim of optimized intervention at this stage would be to try to induce the tolerogenic environment in the gut and also to promote tolerance acquisition.
Fiber is one option and I am involved in one study where dietary fiber is given in pregnancy to improve maternal gut microbiota with possible effects on a later colonization and immune outcomes in their children. Pre-, pro- and synbiotics are still of interest and it will be highly interesting to disentangle the effects of HMOs in tolerance acquisition in the human setting. In addition, we are becoming increasingly aware that environmental microbes also have a huge impact on our microbiota thereby giving rise to other interventions than diet.
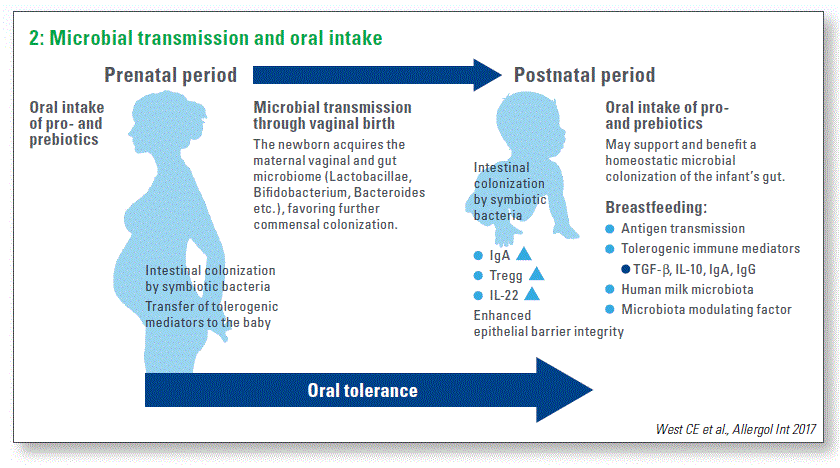
I think that during the last two years, we have come a little bit closer toward understanding this process. We have to take into account the prenatal and postnatal period in order to promote establishment of oral tolerance (Chart 2).
Summary
- Our commensal gut microbiota is im port for the development of immune tolerance
- Dysbiosis is implicated in allergic diseases; more studies investigating the functional aspects of the gut microbiota are needed
- The gut microbiota is tunable
- Gut microbiota modulation strategies should aim to optimize both maternal and infant colonization patterns
