Age-appropriate microbiome maturation and the role of HMO
Age appropriate microbiome maturation and the role of HMO
Olga Sakwinska
Specific features of infant microbiota
Infant microbiota dynamically develops during the first weeks and months of life. Bacterial diversity generally increases with age, accompanied by changes in microbiota composition. Microbiota consists of numerous taxa; this complexity can be described as “microbiota types” where samples with similar microbiota composition are clustered using complex algorithms. The concept of microbiota types was first developed for adult gut microbiota as “enterotypes”. At the very beginning of life, a large proportion of infants show microbiota type which consist mainly of Enterobacteriaceae. Within few weeks a microbiota types rich in bifidobacteria become dominant. Starting at approximately six months, and triggered by the introduction of complementary foods, a progression towards adult-like much more diverse microbiota types is observed.
What is normal infant microbiota?
A recent meta-analysis including 34 studies delineated healthy development of infant gut microbiota and identified the impact of common disruptors of its development. In Cesarean-section delivered infants the colonization with bifidobacteria shows a significant delay in comparison with these delivered vaginally and Bacteroides displayed clear depression lasting at least until the end of the first year of life. Most of infants were at least partially breastfed for variable periods of time, and this heterogeneity precluded a separate analysis of the effect of the feeding mode on microbiota. Taking into consideration the number of studies and individual samples included in this meta-analysis, as well as geographic coverage, the findings can be considered as generally applicable.
Where does infant gut microbiota come from?
Because there are clear differences between microbiota of Cesarean section- and vaginally delivered infants, the concept that vaginal microbiota of the mother is the source of seeding for the infant gut became quite popular. However, recent carefully conducted studies showed that the contribution of the vaginal microbiota of the mother to the gut microbiota of the infant is quite small. Moreover, several studies published in 2018 clearly demonstrate that it is the microbiota of maternal gut that is an import- ant source of infant gut microbiota. In particular, Bacteroides and bifidobacteria were the taxa with clearest contribution of maternal strains to infant gut. Importantly, strain sharing was observed only in vaginally delivered infants, and Cesarean section resulted in dramatic disruption of the maternal seeding of the infant gut. This was observed in two different cohorts from Sweden and from USA. Of course, Cesarean section implies antibiotic usage, at least in the mother, so it is inherently difficult to separate the effects of transfer disruption by the delivery procedure from disruption induced by antibiotics.
Microbiota and later health
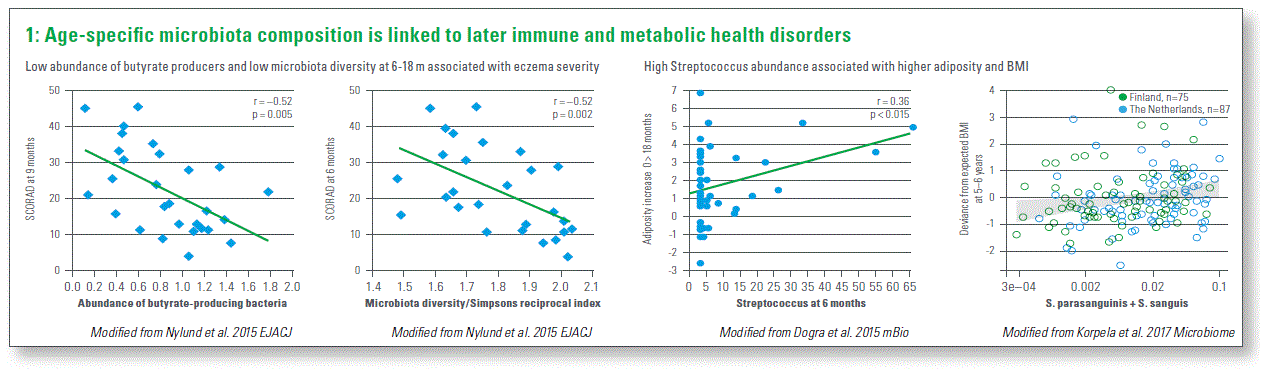
Is the disruption of age-specific microbiota composition linked to later immune and metabolic health disorders? The evidence continues to accumulate and most compelling examples are related to broadly understood immune disorders. For example, microbiota diversity and the abundance of butyrate producers measured at six months of age (the period when they become more abundant in infant microbiota) was associated with decreased eczema scores 3 months later. Another example where similar associations were observed in two independent studies relates to metabolic health outcomes. The abundance of Streptococcus at 6 months was positively correlated with the adiposity increase between birth and 18 months in a cohort of Singaporean infants. An independent study including infants from Finland and the Netherlands reported that the abundance of two Streptococcus species at three months positively correlated with higher than expected BMI at the age of 5 to 6 years (Chart 1).
What are the drivers of microbiota development?
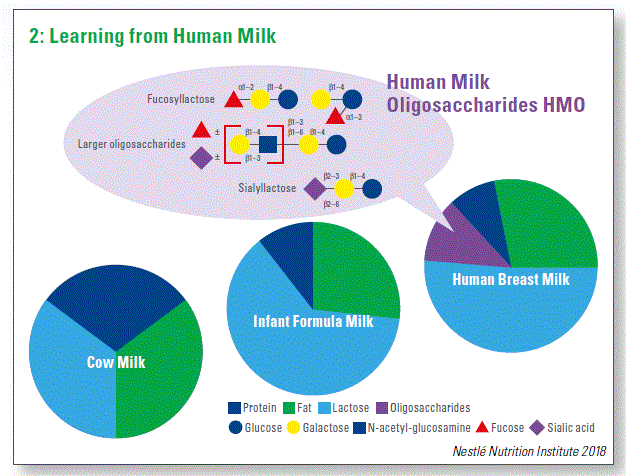
Human milk is the optimal nutrition for infants. What follows, the best way to learn how to improve infant nutrition is from human milk. In particular, human milk oligosaccharides (HMO) are the third largest component of human milk by dry weight. Human milk is unique in terms of HMO quantity (typically>10g/L) and diversity (over 200 structures), which is suggestive of their importance for infant nutri- tion. Despite high diversity, some compounds are predominant, for example 2 fucosyllactose (2’FL) constitutes a large proportion of HMO in human milk. Surprisingly, HMO cannot be directly used by human body, rising questions about their biological functions. On the other hand, HMO are accessible to bacteria. How HMO shape microbiota composition and function is the topic of active research and it is one of the leading hypotheses regarding the influence of HMO on infant health outcomes.
The quantities of HMO in human milk vary among individuals and depend largely on the milk group and the time of lactation. Milk groups are genetically determined. The best understood is the variability in fucosyltransferase 2 gene (FUT2). Approximately 20% of the individuals do not have the functional variant of FUT2 leading to the lack of fucosylated compounds, such as 2’FL, in milk (so called “Non-Secretors”) (Chart 2). Time of lactation is also an important modifier of HMO composition. For example, the amount of 2’FL is high at birth and decreases with the time of lactation, suggesting that such changes could be the driver of the development of infant microbiota.
The fact that 2’FL and other fucosylated compounds are entirely absent from milk of FUT2 negative women allows to link HMO composition in milk with the health outcomes of the infant. In a prospective cohort study conducted in Bangladesh, Dhaka, where most infants were exclusively breastfed until six months, we have observed that infants of FUT negative (Non-Secretor) mothers had increased risk of respiratory infections in the first six months of life. This suggested that 2’FL and fucosylated compounds had protective effect from respiratory infections.
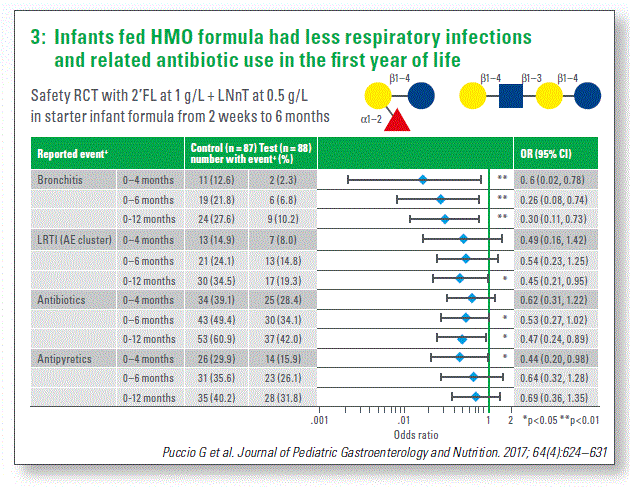
Further, a randomized double-blinded clinical trial compared feeding formula with two HMO, 2’FL and LNnT, with standard formula. Reference group of breasted infants was also included. With the safety as the primary outcome, it was observed that formula with the two HMO was well-tolerated and supported appropriate growth. In addition, as secondary outcome, a decreased risk of respiratory infections, in particular more severe infections and reduced antibiotics and antipyretics use was reported in the group receiving formula with HMO (Chart 3). HMO are hypothesized to have a role in shaping infant gut microbiota. Accordingly, the analysis of gut microbiota at three months of age revealed that HMO increased the proportion of specific microbiota type present in breastfed infants and absent in the group fed standard formula. Interestingly, this shift was also associated with decreased antibiotic usage during the first year of life, as the infants with breasted-specific microbiota type required less frequent antibiotics treatment (50% reduction).
Conclusion
- Gut microbiome develops dynamically in the first months and years of life.
- Transfer of bifidobacteria and Bacteroides from maternal gut is essential for the seeding of normal infant gut microbiota and it is severely disrupted by cesarean section.
- Ageappropriate microbiome maturation is linked to optimal immune development.
- Clinical intervention trial and observational data suggest that 2’Fuco sylHMO provides protection against respiratory infections and decreases the need for antibiotic use.
- The protection appears to be mediated through support of ageappropriate microbiome development by HMO.
