Truths, Myths and Needs of Special Diets: Attention-Deficit/Hyperactivity Disorder, Autism, Non-Celiac Gluten Sensitivity, and Vegetarianism
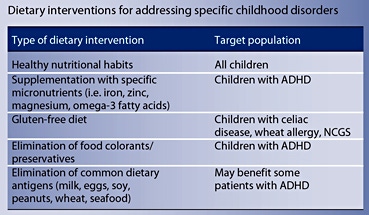
- Healthy and balanced nutrition should be encouraged in children with attention-deficit/hyperactivity disorder (ADHD) and autism. In children with ADHD, dietetic restriction of sugar, sweeteners and elimination of colorants/preservatives improve behavioral and attention performance. Other specific elimination diets should only be recommended to children with demonstrated food allergy. Supplementation with omega-3 fatty acids improves behavior.
- A gluten- and casein-free diet does not have strong evidence supporting its indication in the management of autism. An exclusion diet is only indicated in children with demonstrated milk and/or wheat allergy. Macro- and micronutrient deficiencies have been described in children under this diet, and health professional supervision should be encouraged.
- A new entity, non-celiac gluten sensitivity, with a still evolving definition and clinical spectrum, has been described. The benefits of a gluten-free diet (GFD) are clearly supported in these conditions. Until now, no long-term complication has been described in patients not adhering strictly to this diet.
- GFD without health professional supervision has risks of vitamin (mainly B vitamins and folic acid) and micronutrient (especially iron and zinc) deficiencies as well as lower fiber intake.
- Subjects on a vegetarian diet, especially vegans, are at risk of vitamin B 12 deficiency if they are not adequately supplemented.
- A vegetarian diet is a feasible alternative if implemented with supervision by a specialist, especially during vulnerable periods of life.
Education on Healthy Nutritional Habits
Excess of sugar and sweetener consumption has been associated, although not consistently, with hyperactive and disruptive behavior in ADHD patients [11–13]. Few interventional studies have been performed comparing healthy food against fast food/highly sweetened food. In a cross-sectional study, Park et al. [14] described an association between intake of sweetened desserts, fried food, salt and a higher inattention and hyperactivity score in ADHD school-age children compared with a balanced diet. In addition, Ghanizadeh et al. [15] reported in a randomized controlled trial (RCT) an association between improvement of inattention score and an increase consumption of ‘healthier’ foods. Although more evidence is necessary, the expert recommendation based on very limited research is to indicate a balanced and ‘healthy’ diet for children with ADHD considering its proven beneficial effects in global wellbeing and potential additional benefits for cognition and behavior.
Supplementation
Open-label trials and RCTs to determine the effect of iron, zinc and magnesium supplementation on inattention and hyperactivity behavior in patients with ADHD have been performed [7, 16–24]. Although some of these studies suggest a beneficial effect, especially in children with confirmed deficits, a recent systematic review concluded that current evidence is still inconclusive [16]. The expert recommendation is to treat patients with demonstrated micronutrient deficiencies and, in children who do not ingest a balanced diet and/or have stimulant-medication- related appetite suppression, to supplement with multivitamins/minerals [21]. This is based on the fact that a recommended daily dose of micronutrient intake carries little risk [10].
A meta-analysis has shown that children with ADHD have a lower plasma concentration of omega-3 fatty acids than controls [25]. However, whether this observation contributes to ADHD pathophysiology or is a casual finding has not yet been demonstrated. A recent meta-analysis including 10 RCTs of omega-3 fatty acid supplementation did not find positive effects on ADHD symptoms. However, subgroup analyses of higher-quality studies found a significant reduction in emotional lability and oppositional behavior [26]. The authors concluded that current evidence supports only a small beneficial effect of omega-3 fatty acids on some behavioral symptoms [25, 26].
Elimination Diets
The hypothesis of the effect of synthetic food colorants on hyperactivity in ADHD patients was first introduced in the 1970s by Feingold [19, 27]. Either allergenic or pharmacologic mechanisms were suggested, and elimination diet was proposed as an adjuvant treatment for hyperactivity symptoms [2]. Most of the studies are openlabel, non-blinded trials including few patients, with a wide heterogeneity in outcome definition, and have generated inconclusive results [2, 28]. RCTs with exclusion and challenge with colorants have demonstrated improvement of hyperactivity in 10–30% of patients [29– 31]. A meta-analysis of RCTs with colorant elimination diet suggests a modest but consistent effect in non-selected ADHD patients [32–34]. This percentage may even increase when the intervention is applied to selected patients with symptoms suggestive of food allergy [2]. More restrictive diets excluding milk, eggs, soy, peanuts, wheat and seafood as well as an ‘oligoantigenic diet’ have also been tried in RCTs, and results have been promising with a significant decrease in hyperactive behaviors in up to 64% of children [30, 35, 36]. Taken together, these studies suggest that elimination diets can benefit a subgroup of ADHD patients, although research directed to identify the best candidates for this intervention is needed.
In the last two decades, a current promoting GFD more widely to the general population has emerged and spread through social media
NCGS is a syndrome characterized by intestinal and extra-intestinal symptoms related to the ingestion of gluten- containing food in subjects that are not affected by either celiac disease or wheat allergy [43, 44] . Epidemiological studies in developed countries have reported a prevalence of 0.6–6% [39, 45, 46] . This frequency increases up to 30% in patients evaluated for irritable bowel syndrome
[47, 48]. NCGS seems to be a multifactorial condition with a genetic background and environmental triggers including ingested grain proteins. An innate immune response has been implicated in its pathogenesis, although the exact mechanisms remain unclear [49]. In addition to gluten, other prolamins and amylase trypsin inhibitor have been identified as triggers of symptoms in patients with NCGS [37]. The most frequent symptoms in such patients are abdominal pain (80%), chronic diarrhea (73%), fatigue (33%) and bloating (26%), which commonly overlap with those of irritable bowel syndrome. Other presentations are eczema, migraine, blurry vision, depression, anemia, limb paresthesias and arthralgia [37, 40]. Although anti-gliadin antibodies have been reported to be elevated more frequently in NCGS patients than in healthy controls, no reliable biomarker is currently available, and diagnosis confirmation relies on clinical response to a period of exclusion diet (GFD) followed by gluten challenge [40]. Benefits of a GFD have been well documented in patients with NCGS [37]. Until now, no long-term complications have been described for this condition, therefore, adherence to a GDF does not have to be as strict as in celiac disease and wheat allergy but rather symptom adjusted [39, 49].
The number of people who adopt a vegetarian diet grows permanently. In Europe, around 2–5% of the population is vegetarian and in the United States, 2% of teenagers follow this type of diet, with 0.5% of them being vegan [42].
The American Association of Nutritionists states that ‘well planned vegan, lacto-vegetarian and ovo-lacto-vegetarian diets are appropriate for any stage of the life cycle, including pregnancy and lactation’. However, the association also indicates that, in special situations (as when dealing with children or teenagers), the support of a nutritionist is recommended [43].
Health Effects: Pros, Cons and Recommendations of Vegetarian Diets
Past research focused on vegetarian adults has shown that this segment of the population has a lower body mass index (BMI), total cholesterol, LDL cholesterol and glycemic levels, when compared to their omnivorous counterparts [44]. Prospective cohort studies have also shown that, in comparison to a regular diet, a vegetarian diet acts as a protective factor for entities such as ischemic cardiopathy mortality (–25% and total cancer (–8%) but not for cardiovascular and cerebrovascular diseases [45]. On the other hand, investigations focused on ovo-lacto vegetarian children have shown that their growth patterns are identical to those of omnivorous children. However, results are different for vegan children who, in general, were slimmer and wore smaller clothing sizes [42].
It is important to emphasize that correlations have been found between followers of vegetarian diets and patients with eating disorders, which is why it is highly recommended to perform a detailed history focused on the reasons behind the diet change, especially when dealing with teenagers [46].
What about Vitamin B 12 ?
Until recently it was assumed that only strict vegetarians (vegans) showed deficiency levels of vitamin B 12; however, a meta-analysis of subjects on different kinds of vegetarian diets showed that all vegetarians displayed deficiency in B 12 levels, regardless of the specific type of diet, demographic characteristics, place of residence or age.
Vitamin B 12 deficiency, in the long run, produces megaloblastic anemia, which leads to increased homocysteine levels, a substance that leads to the development of cardiovascular and cerebrovascular diseases. Studies have shown that in pregnant women, B 12 deficiency is associated with high levels of homocysteine [47, 48]. Around 25–86% of vegetarian children also show such a deficiency, with a higher prevalence amongst vegans [49]. Another study conducted in Finland showed that supplementing this vitamin prevents its deficiency in teenagers [50].
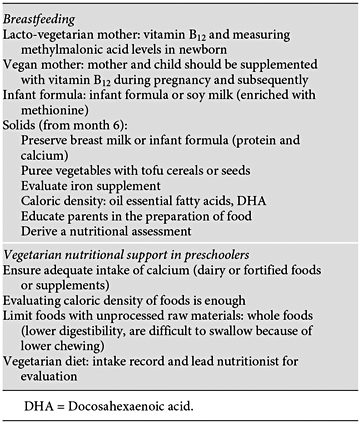
It is fundamental to highlight that newborns that are being exclusively breastfed by their vegan mothers can show a severe deficiency of vitamin B 12 , leading to metabolic acidosis, high levels of methylmalonic acid and ammonia, which in turn could cause damage to the central nervous system, unless the vitamin is appropriately supplemented to the baby [51–53].
Recommendations established by the Recommended Dietary Intake (RDI) are 1.8 μg a day for children between 9 and 13 years of age and 2.5 μg for teenagers between 14 and 18 years of age. The best sources for vitamin B 12 are: bovine liver and meat, clams, fish, chicken, turkey, eggs, dairy, fortified cereals, and nutritional yeasts. Because vegetarians do not consume these kinds of foods, the only way to avoid any complications is by administering supplements such as hydroxocobalamin in doses that range from 1 to 5 mg a day (table 1).
How Does Consuming Low-Biological-Value Protein Affect the Individual?
Protein quality is associated with the type of amino acid that it contains. The highest-quality protein available comes from animal sources: eggs, dairy and meat in general. Protein that comes from legumes is deficient in methionine; however, its quality is improved when blended with other kinds of food, making it comparable to protein of animal origin. Thus, adding cereal (as rice, pasta or seeds) to these meals can be highly beneficial. This is why consumption of mixed foods such as beans and noodles, chickpeas and boiled corn or lentils and rice are an excellent source of good-value proteins in vegetarian diets.
The amount of protein consumption that vegetarians require is somewhat higher than that recommended for the omnivorous population. This is because when a diet is very rich in fiber, the bioavailability of protein is estimated to be only 75%. This means that vegetarians need a protein intake that is at least 1.3 times higher than that recommended for omnivorous subjects [54]. In the case of vegan mothers whose babies are not being breastfed, it is recommended to use infant soy-based formulas that are closer to breastmilk than regular soy milk, as these formulas have added methionine, which helps to improve the quality of the vegetal protein. Soy milk or any milk substitute, cerealbased products, nuts, legumes (rice, oatmeal, etc.) do not cover the nutritional basics for children, as they are deficient in amino acids, vitamins and minerals [55].
In children and teenagers, the consumption of tofu, tempeh, dehydrated soy, etc. combined with cereals is an excellent source of high-value protein but does not provide sufficient quantities of iron or vitamin B 12, which need to be supplemented.
What Is the Status of Bone Mineral Density, Calcium Consumption and Vitamin D?
Calcium and Vitamin D deficiencies have been reported in strict vegetarians; however, no differences have been noted in bone mineral density (BMD) between omnivorous subjects and ovo-lacto vegetarians [56]. Despite this information, not all studies agree that these deficiencies are associated with bone density loss, nor with a higher fracture incidence. Nonetheless, a correlation has been proven to exist between age and lean mass with lower levels of BMD [57]. A longitudinal study in adults showed that there were no abnormalities present in the bone health of vegetarian women [58].
Past research stated that children who are exclusively fed non-supplemented soy milk show no signs of rickets. In addition, it was determined that BMD and fracture risk are similar in ovo-lacto-vegetarian and omnivorous children. However, in vegan children and teenagers, a lower BMD and a higher risk of bone fracture was observed, associated with low levels of calcium consumption.
It is important to state that there are a large number of vegetables, nuts and legumes that are rich in calcium (broccoli, spinach, almonds, beans, etc.), but the presence of oxalates makes the absorption of this mineral deficient [59].
Vitamin D is mainly obtained by exposure to sunlight, and there are very few foods that contain it. Some of them are marine oils, fatty fishes (herring, for instance, contains 1,600 UI or 40 g), liver or aquatic animal fat, such as from seals and polar bears, and eggs from hens that have been fed this vitamin. For this reason, a large portion of vitamin D that is ingested by teenagers comes from fortified foods such as dairy or cereal.
Iron and Zinc Deficiencies
Despite the large consumption of non-heme iron found in green leafy vegetables, which has a lower bioavailability than the heme iron found in red meat, iron deficiency is not commonly found in vegetarians, as their consumption of cereals, legumes, nuts, seeds, fortified foods and food rich in vitamin C favors non-heme iron absorption and also counteracts the inhibitor effects that phytates have on absorbing this mineral [60, 61].
It is know that phytates affect zinc’s bioavailability, which in turn could produce deficiencies in vegetarians [62]. A meta-analysis conducted on pregnant women found significant differences in this mineral ingestion when compared to non-vegetarian women. However, no differences were found in their plasma or serum levels. Further studies are suggested to determine whether physiological adaptations in the absorption of this mineral exist [63].
A number of pharmacological treatments and dietary adaptations have been created in order to make improvements in the sensory and behavioral aspects of these conditions. One of the dietary treatments used is gluten- (wheat and cereals) and casein- (milk and derivatives) free diet, which has been associated with improvements in learning processes. Many studies have evaluated its effects, but none of them have been controlled or doubleblind [65–67]. A single-blinded study that focused only on 10 cases was carried out and showed that gluten and casein elimination resulted in improvements in communication and language, although these effects could not be directly associated with a dietary change, due to the very small sample size and the short evaluation period of only one year [68].
Another study, in which parents of autistic children with special diets were surveyed, showed that 20–29% of parents mentioned significant improvements in relation to their children’s condition [69]. However, studies on the impact of these diets on other aspects associated with autism, such as gastrointestinal disorders, attention and concentration deficits, are non-conclusive.
It is important to state that the implementation of a diet without nutritional and medical control can cause very specific deficiencies. A study conducted in Spain showed that a gluten- and casein-free diet resulted in weight loss and a lower BMI, as well as a lower intake of essential nutrients (like phosphorus and calcium among others), but an appropriate intake of legume fiber and vegetables [70]. However, vitamin D supplementation is recommended, as well as an evaluation of the long-term nutritional and behavioral effects. One case of vitamin deficiency-induced xerophthalmia was observed in an autistic patient following a GFD [71].
The exposure to a gluten- and casein-free diet for a week did neither affect the maladaptive behavior nor the intensity of the gastrointestinal symptoms or the urinary excretion of the fatty acid-binding protein (I-FABP) in autistic children [72]. More long-term studies to evaluate the physiopathological mechanisms of the enterocyte in autistic children are needed.
A gluten- and casein-free diet should only be implemented if an allergy or intolerance to gluten or milk is diagnosed
Most of the research that evaluates the effectiveness of a gluten- and casein-free diet in autistic children presents serious methodological problems. The evidence that is shown to support the therapeutic value of this diet is limited and weak. A gluten- and casein-free diet should only be implemented if an allergy or intolerance to gluten or milk is diagnosed [69].
The scientific evidence of the use of a diet free of gluten and casein in the treatment of autism is weak and poor. It is proposed that a diet free of gluten and casein should only be administered if a food allergy or gluten intolerance is diagnosed. GFD is indicated for the treatment of celiac disease, wheat allergy and NCGS. This diet has to be implemented under professional supervision to avoid imbalanced ingestion.
Vegetarianism does not pose any nutritional threat as it includes egg and milk. With adequate supplementation of calcium, vitamin B 12 and other micronutrients as well as under professional supervision, children will receive all necessary nutrients. On the other hand, vegan diets are not recommended at any age, vegetarian diet is a feasible alternative if implemented with supervision by a specialist, especially during vulnerable periods of life.
The writing of this article was supported by Nestlé Nutrition Institute.
- Thapar A, Cooper M: Attention deficit hyperactivity disorder. Lancet 2016;387:1240–1250.
- Nigg JT, Holton K: Restriction and elimination diets in ADHD treatment. Child Adolesc Psychiatr Clin N Am 2014; 23: 937–953.
- Klein RG, Landa B, Mattes JA, Klein DF: Methylphenidate and growth in hyperactive children. A controlled withdrawal study. Arch Gen Psychiatry 1988; 45: 1127–1130.
- Coyle JT: Psychotropic drug use in very young children. JAMA 2000; 283: 1059–1060.
- Nasrallah HA, Loney J, Olson SC, McCalley- Whitters M, Kramer J, Jacoby CG: Cortical atrophy in young adults with a history of hyperactivity in childhood. Psychiatry Res 1986; 17: 241–246.
- Toren P, Eldar S, Sela BA, Wolmer L, Weitz R, Inbar D, et al: Zinc deficiency in attentiondeficit hyperactivity disorder. Biol Psychiatry 1996; 40: 1308–1310.
- Cortese S, Angriman M, Lecendreux M, Konofal E: Iron and attention deficit/hyperactivity disorder: what is the empirical evidence so far? A systematic review of the literature. Expert Rev Neurother 2012; 12: 1227–1240.
- Cortese S, Angriman M: Attention-deficit/ hyperactivity disorder, iron deficiency, and obesity: is there a link? Postgrad Med 2014; 126: 155–170.
- Curtis LT, Patel K: Nutritional and environmental approaches to preventing and treating autism and attention deficit hyperactivity disorder (ADHD): a review. J Altern Complement Med 2008; 14: 79–85.
- Hurt EA, Arnold LE: An integrated dietary/ nutritional approach to ADHD. Child Adolesc Psychiatr Clin N Am 2014; 23: 955–964.
- Johnson RJ, Gold MS, Johnson DR, Ishimoto T, Lanaspa MA, Zahniser NR, et al: Attention- deficit/hyperactivity disorder: is it time to reappraise the role of sugar consumption? Postgrad Med 2011; 123: 39–49.
- Azadbakht L, Esmaillzadeh A: Dietary patterns and attention deficit hyperactivity disorder among Iranian children. Nutrition 2012; 28: 242–249.
- Wolraich ML, Lindgren SD, Stumbo PJ, Stegink LD, Appelbaum MI, Kiritsy MC: Effects of diets high in sucrose or aspartame on the behavior and cognitive performance of children. N Engl J Med 1994; 330: 301–307.
- Park S, Cho SC, Hong YC, Oh SY, Kim JW, Shin MS, et al: Association between dietary behaviors and attention-deficit/hyperactivity disorder and learning disabilities in school-aged children. Psychiatry Res 2012; 198: 468–476.
- Ghanizadeh A, Haddad B: The effect of dietary education on ADHD, a randomized controlled clinical trial. Ann Gen Psychiatry 2015; 14: 12.
- Hariri M, Azadbakht L: Magnesium, iron, and zinc supplementation for the treatment of attention deficit hyperactivity disorder: a systematic review on the recent literature. Int J Prev Med 2015; 6: 83.
- Hawkey E, Nigg JT: Omega-3 fatty acid and ADHD: blood level analysis and meta-analytic extension of supplementation trials. Clin Psychol Rev 2014; 34: 496–505.
- Cooper RE, Tye C, Kuntsi J, Vassos E, Asherson P: The effect of omega-3 polyunsaturated fatty acid supplementation on emotional dysregulation, oppositional behaviour and conduct problems in ADHD: a systematic review and meta-analysis. J Affect Disord 2016; 190: 474–482.
- Feingold BF: Hyperkinesis and learning disabilities linked to artificial food flavors and colors. Am J Nurs 1975; 75: 797–803.
- Pelsser LM, Buitelaar JK, Savelkoul HF: ADHD as a (non) allergic hypersensitivity disorder: a hypothesis. Pediatr Allergy Immunol 2009; 20: 107–112.
- Rowe KS, Rowe KJ: Synthetic food coloring and behavior: a dose response effect in a double- blind, placebo-controlled, repeatedmeasures study. J Pediatr 1994; 125: 691–698.
- Pelsser LM, Frankena K, Toorman J, Savelkoul HF, Dubois AE, Pereira RR, et al: Effects of a restricted elimination diet on the behaviour of children with attention-deficit hyperactivity disorder (INCA study): a randomised controlled trial. Lancet 2011; 377: 494–503.
- McCann D, Barrett A, Cooper A, Crumpler D, Dalen L, Grimshaw K, et al: Food additives and hyperactive behaviour in 3-year-old and 8/9-year-old children in the community: a randomised, double-blinded, placebo-controlled trial. Lancet 2007; 370: 1560–1567.
- Nigg JT, Lewis K, Edinger T, Falk M: Metaanalysis of attention-deficit/hyperactivity disorder or attention-deficit/hyperactivity disorder symptoms, restriction diet, and synthetic food color additives. J Am Acad Child Adolesc Psychiatry 2012; 51: 86–97.e8.
- Schab DW, Trinh NH: Do artificial food colors promote hyperactivity in children with hyperactive syndromes? A meta-analysis of double-blind placebo-controlled trials. J Dev Behav Pediatr 2004; 25: 423–434.
- Sonuga-Barke EJ, Brandeis D, Cortese S, Daley D, Ferrin M, Holtmann M, et al: Nonpharmacological interventions for ADHD: systematic review and meta-analyses of randomized controlled trials of dietary and psychological treatments. Am J Psychiatry 2013; 170: 275–289.
- Egger J, Carter CM, Graham PJ, Gumley D, Soothill JF: Controlled trial of oligoantigenic treatment in the hyperkinetic syndrome. Lancet 1985; 1: 540–545.
- Schmidt MH, Möcks P, Lay B, Eisert HG, Fojkar R, Fritz-Sigmund D, et al: Does oligoantigenic diet influence hyperactive/conduct- disordered children – a controlled trial. Eur Child Adolesc Psychiatry 1997; 6: 88–95.
- Fasano A, Sapone A, Zevallos V, Schuppan D: Nonceliac gluten sensitivity. Gastroenterology 2015; 148: 1195–1204.
- Gasbarrini G, Mangiola F: Wheat-related disorders: a broad spectrum of ‘evolving’ diseases. United European Gastroenterol J 2014; 2: 254–262.
- Sapone A, Bai JC, Ciacci C, Dolinsek J, Green PH, Hadjivassiliou M, et al: Spectrum of gluten- related disorders: consensus on new nomenclature and classification. BMC Med 2012; 10: 13.
- Catassi C, Elli L, Bonaz B, Bouma G, Carroccio A, Castillejo G, et al: Diagnosis of nonceliac gluten sensitivity (NCGS): the Salerno experts’ criteria. Nutrients 2015; 7: 4966– 4977.
- Volta U, Caio G, Tovoli F, De Giorgio R: Nonceliac gluten sensitivity: questions still to be answered despite increasing awareness. Cell Mol Immunol 2013; 10: 383–392.
- See JA, Kaukinen K, Makharia GK, Gibson PR, Murray JA: Practical insights into gluten- free diets. Nat Rev Gastroenterol Hepatol 2015; 12: 580–591.
- Tonutti E, Bizzaro N: Diagnosis and classification of celiac disease and gluten sensitivity. Autoimmun Rev 2014; 13: 472–476.
- Ludvigsson JF, Leffler DA, Bai JC, Biagi F, Fasano A, Green PH, et al: The Oslo definitions for coeliac disease and related terms. Gut 2013; 62: 43–52.
- DiGiacomo DV, Tennyson CA, Green PH, Demmer RT: Prevalence of gluten-free diet adherence among individuals without celiac disease in the USA: results from the Continuous National Health and Nutrition Examination Survey 2009–2010. Scand J Gastroenterol 2013; 48: 921–925.
- Volta U, Bardella MT, Calabrò A, Troncone R, Corazza GR: Sensitivity SGfN-CG. An Italian prospective multicenter survey on patients suspected of having non-celiac gluten sensitivity. BMC Med 2014; 12: 85.
- Carroccio A, Mansueto P, Iacono G, Soresi M, D’Alcamo A, Cavataio F, et al: Non-celiac wheat sensitivity diagnosed by double-blind placebo-controlled challenge: exploring a new clinical entity. Am J Gastroenterol 2012; 107: 1898–1906; quiz 907.
- Biesiekierski JR, Newnham ED, Irving PM, Barrett JS, Haines M, Doecke JD, et al: Gluten causes gastrointestinal symptoms in subjects without celiac disease: a double-blind randomized placebo-controlled trial. Am J Gastroenterol 2011; 106: 508–514; quiz 15.
- Catassi C, Bai JC, Bonaz B, Bouma G, Calabrò A, Carroccio A, et al: Non-Celiac Gluten sensitivity: the new frontier of gluten related disorders. Nutrients 2013; 5: 3839–3853.
- Van Winckel M, Vande Velde S, De Bruyne R, Van Biervliet S: Clinical practice: vegetarian infant and child nutrition. Eur J Pediatr 2011; 170: 1489–1494.
- Craig WJ, Mangels AR; American Dietetic Association. Position of the American Dietetic Association: vegetarian diets. J Am Diet Assoc 2009; 109: 1266–1282.
- Chiu YF, Hsu CC, Chiu TH, Lee CY, Liu TT, Tsao CK, Chuang SC, Hsiung CA: Cross-sectional and longitudinal comparisons of metabolic profiles between vegetarian and nonvegetarian subjects: a matched cohort study. Br J Nutr 2015; 114: 1313–1320.
- Dinu M, Abbate R, Gensini GF, Casini A, Sofi F: Vegetarian, vegan diets and multiple health outcomes: a systematic review with meta-analysis of observational studies. Crit Rev Food Sci Nutr 2016, Epub ahead of print.
- Van Winckel M, Vande Velde S, De Bruyne R, Van Biervliet S: Clinical practice: vegetarian infant and child nutrition. Eur J Pediatr 2011; 170: 1489–1494.
- Piccoli GB, Clari R, Vigotti FN, Leone F, Attini R, Cabiddu G, Mauro G, Castelluccia N, Colombi N, Capizzi I, Pani A, Todros T, Avagnina P: Vegan-vegetarian diets in pregnancy: danger or panacea? A systematic narrative review. BJOG 2015; 122: 623–633.
- Gadgil MS, Joshi KS, Naik SS, Pandit AN, Otiv SR, Patwardhan B: Association of homocysteine with global DNA methylation in vegetarian Indian pregnant women and neonatal birth anthropometrics. J Matern Fetal Neonatal Med 2014; 27: 1749–1753.
- Pawlak R, Parrott SJ, Raj S, Cullum-Dugan D, Lucus D: How prevalent is vitamin B(12) deficiency among vegetarians? Nutr Rev 2013; 71: 110–117.
- Elorinne AL, Alfthan G, Erlund I, Kivimäki H, Paju A, Salminen I, Turpeinen U, Voutilainen S, Laakso J: Food and nutrient intake and nutritional status of finnish vegans and non-vegetarians. PLoS One 2016; 11: e0148235.
- Goraya JS, Kaur S, Mehra B: Neurology of nutritional vitamin B12 deficiency in infants: case series from India and literature review. J Child Neurol 2015; 30: 1831–1837.
- Kocaoglu C, Akin F, Caksen H, Böke SB, Ars lan S, Aygün S: Cerebral atrophy in a vitamin B12-deficient infant of a vegetarian mother. J Health Popul Nutr 2014; 32: 367– 371.
- Guez S, Chiarelli G, Menni F, Salera S, Principi N, Esposito S: Severe vitamin B12 deficiency in an exclusively breastfed 5-monthold Italian infant born to a mother receiving multivitamin supplementation during pregnancy. BMC Pediatr 2012; 12: 85.
- Gilsing A, Weijenberg M, Goldbohm A, Dagnelie P, van den Brandt P, Schouten L: The Netherlands Cohort Study – Meat Investigation Cohort; a population-based cohort over-represented with vegetarians, pescetarians and low meat consumers. Nutr J 2013; 12: 156.
- Le Louer B, Lemale J, Garcette K, Orzechowski C, Chalvon A, Girardet JP, Tounian P: Severe nutritional deficiencies in young infants with inappropriate plant milk consumption (in French). Arch Pediatr 2014; 21: 483–488.
- Tucker KL: Vegetarian diets and bone status. Am J Clin Nutr 2014; 100(suppl 1):329S– 335S.
- Knurick JR, Johnston CS, Wherry SJ, Aguayo I: Comparison of correlates of bone mineral density in individuals adhering to lacto-ovo, vegan, or omnivore diets: a cross-sectional investigation. Nutrients 2015; 7: 3416–3426.
- Ho-Pham LT, Vu BQ, Lai TQ, Nguyen ND, Nguyen TV: Vegetarianism, bone loss, fracture and vitamin D: longitudinal study in Asian vegans and non-vegans. Eur J Clin Nutr 2012; 66: 75–82.
- Mangels AR: Bone nutrients for vegetarians. Am J Clin Nutr 2014; 100(suppl 1):469S– 475S.
- Saunders AV, Craig WJ, Baines SK, Posen JS: Iron and vegetarian diets. Med J Aust 2013; 199(suppl 4):S11–S16.
- Gibson RS, Heath AL, Szymlek-Gay EA: Is iron and zinc nutrition a concern for vegetarian infants and young children in industrialized countries? Am J Clin Nutr 2014; 100(suppl 1):459S–468S.
- Foster M, Samman S: Vegetarian diets across the lifecycle: impact on zinc intake and status. Adv Food Nutr Res 2015; 74: 93–131.
- Foster M, Herulah UN, Prasad A, Petocz P, Samman S: Zinc status of vegetarians during pregnancy: a systematic review of observational studies and meta-analysis of zinc intake. Nutrients 2015; 7: 4512–4525.
- Frohna JG: Toward better evidence for parent training programs for autism spectrum disorder. J Pediatr 2005; 147: 283–284.
- Reichelt KL, Ekrem J, Scott H: Gluten, milk proteins and autism: dietary intervention effects on behaviour and peptide secretion. J Appl Nutr 1990; 42: 1–1.
- Lucarelli S, Frediani T, Zingoni A, Ferruzzi F, Giardini O, Quintieri F, Barbato M, D’Eufemia P, Cardi E: Food allergy and infantile autism. Panminerva Med 1995; 37: 137–141.
- Whiteley P, Rodgers J, Savery D, Shattock P: A gluten-free diet as an intervention for autism and associated spectrum disorders: preliminary findings. Autism 1999; 3: 45–65.
- Knivsberg AM, Reichelt KL, Høien T, Nødland M: A randomised, controlled study of dietary intervention in autistic syndromes. Nutr Neurosci 2002; 5: 251–261.
- Lange KW, Hauser J, Reissmann A: Glutenfree and casein-free diets in the therapy of autism. Curr Opin Clin Nutr Metab Care 2015; 18: 572–575.
- Marí-Bauset S, Llopis-González A, Zazpe I, Marí-Sanchis A, Suárez-Varela MM: Nutritional impact of a gluten-free casein-free diet in children with autism spectrum disorder. J Autism Dev Disord 2016; 46: 673–684.
- Chiu M, Watson S: Xerophthalmia and vitamin A deficiency in an autistic child with a restricted diet. BMJ Case Rep 2015; 2015: pii:bcr2015209413.
- Pusponegoro HD, Ismael S, Firmansyah A, Sastroasmoro S, Vandenplas Y: Gluten and casein supplementation does not increase symptoms in children with autism spectrum disorder. Acta Paediatr 2015; 104:e500–e505.