Researchers find gene that makes some infants susceptible to respiratory and gastrointestinal illness
New research published in October 2018 has discovered that multiple genetic variants within the FUT2 gene make some infants especially susceptible to respiratory and gastrointestinal illness.
FUT2 Genetic Variants and Reported Respiratory and Gastrointestinal Illnesses During Infancy
Sheila J. Barton,1 Robert Murray,2 Karen A. Lillycrop,2,4 Hazel M. Inskip,1,3 Nicholas C. Harvey,1,3 Cyrus Cooper,1,3 Neerja Karnani,5,6 Irma Silva Zolezzi,7 Norbert Sprenger,7 Keith M. Godfrey,1,2,3 and Aristea Binia7
1MRC Lifecourse Epidemiology Unit and 2Human Development and Health Academic Unit, University of Southampton, 3NIHR Southampton Biomedical Research Centre, University of Southampton and University Hospital Southampton NHS Foundation Trust, and 4School of Biological Sciences, University of Southampton, Southampton General Hospital, United Kingdom; 5Singapore Institute for Clinical Sciences, A*STAR, and 6Department of Biochemistry, Yong Loo Lin School of Medicine, National University of Singapore; and 7Nestlé Research Center, Nestec, Lausanne, Switzerland
Background. Fucosyltransferase 2 (FUT2) controls the production of digestive and respiratory epithelia of histo-blood group antigens involved in the attachment of pathogens. The aim of our study was to relate FUT2 variants to reported gastrointestinal and respiratory illnesses in infancy.
Methods. In the Southampton Women’s Survey, FUT2 genetic variants (single-nucleotide polymorphisms [SNPs] rs601338 and rs602662) were genotyped in 1831 infants and related to infant illnesses, after adjustment for sex, breastfeeding duration, and potential confounders.
Results. For FUT2 SNP rs601338, the risk ratios for =1 bout of diarrhea during ages 6–12 months and ages 12–24 months per additional risk (G) allele were 1.23 (95% confidence interval [CI], 1.08–1.4; P = .002) and 1.41 (95% CI, 1.24–1.61; P = 1.7 × 10-7), respectively; the risk ratio for =1 diagnosis of a lower respiratory illness (ie, pneumonia or bronchiolitis) during ages 12–24 months per additional G allele was 2.66 (95% CI, 1.64–4.3; P = .00007). Similar associations were found between rs602662 and gastrointestinal and respiratory illnesses, owing to the high linkage disequilibrium with rs601338 (R2 = 0.92). Longer breastfeeding duration predicted a lower risk of diarrhea, independent of infant FUT2 genotype.
Conclusions. We confirmed that FUT2 G alleles are associated with a higher risk of infant gastrointestinal illnesses and identified novel associations with respiratory illnesses. FUT2 locus variants need consideration in future studies of gastrointestinal and respiratory illnesses among infants.
Keywords. FUT2 variants; gastrointestinal and respiratory illnesses; pediatric illnesses.
Received 6 July 2018; editorial decision 21 September 2018; accepted 25 October 2018; published online October 30, 2018.
Presented in part: FUT2 gene variants are associated with reported gastrointestinal and respiratory illnesses during infancy in the Southampton Women’s Survey (SWS). International Society for Developmental Origins of Health and Disease World Conference, Rotterdam, the Netherlands, 18 October 2017. Poster PO3.02.13.
Correspondence: A. Binia, PhD, Nestlé Research Center, PO Box 44, Vers-chez-les-Blanc, CH-1000 Lausanne 26 (aristea.binia@rdls.nestle.com).
The Journal of Infectious Diseases® 2018;XXXX:1–8
© Crown copyright 2018. This article contains public sector information licensed under the Open Government Licence v3.0 (http://www.nationalarchives.gov.uk/doc/open-government-licence/version/3/). DOI: 10.1093/infdis/jiy582
Diarrhea and respiratory illnesses are major causes of morbidity and mortality in young children aged <5 years of age, with >2 million deaths per year [1]. Preventive measures and improved disease management have reduced mortality, but it remains particularly substantial in low- or middle-income countries [1], accounting for 30% of early childhood deaths worldwide [2]. Mortality is especially high in young children with multiple episodes per year [3, 4]. In developed countries, gastroenteritis cases are mainly due to rotavirus (in unvaccinated children), norovirus, adenovirus, and Salmonella organisms, with the first 2 pathogens accounting for one half and one third of cases, respectively, and the last 2 accounting for approximately 10% of cases. In young children, rotavirus and norovirus infections are highly prevalent, with a peak age of occurrence of 3 months and 2 years, respectively [5]. For acute lower respiratory tract infection in young children, human respiratory syncytial virus (RSV) is the most common causal pathogen [6]. Breastfeeding is proposed as a protective factor against both diarrhea and respiratory infections, suggesting that early cessation of lactation and the benefits delivered to the infants might contribute to avertable infections [7, 8].
Host characteristics are known to impact the susceptibility to early childhood infections. Recent studies have reported that genetic polymorphisms affecting the production of histo-blood group antigens (HBGAs), which can act as attachment sites for specific pathogens, are associated with the incidence of diarrhea [9–11]. FUT2 (OMIM number +182100) encodes a1,2-fucosyltransferase 2 (FUT2), which catalyzes the addition of a fucose to the H-type 1 precursor, generating the H-type antigen in saliva and on digestive and respiratory epithelia, producing the secre-tor phenotype. Inactivating polymorphisms in FUT2 give rise to the nonsecretor status, characterized by the absence of H-type antigen in mucosal tissues and secretions [12]. The objective of our study was to assess the genetic risk that FUT2 contributes to infant morbidities in a United Kingdom mother-offspring cohort study (the Southampton Women’s Survey [SWS]); given the presence of H-type antigen on respiratory epithelia, our particular focus was on respiratory illnesses that have not previously been related to FUT2 variants in population-based studies.
METHODS
Study Population
Offspring of participants in the SWS were studied [13]. Between 1998 and 2007, 3158 infants were born, and data were collected on them both during the pregnancy and after the birth. Infant health outcomes (chest wheezing, cough, pneumonia or bronchiolitis, croup, diarrhea, vomiting, and ear infections) were assessed by nurse-led questionnaires 6, 12, and 24 months after birth (questions asked by research nurses on infant health outcomes are listed in Supplementary Table 2). Detailed information about breastfeeding was also obtained by nurse-led questionnaires administered 6, 12, and 24 months after the birth of the child. Maternal smoking during pregnancy was assessed by questionnaire in early and late pregnancy. Visits occurred at short intervals (6, 12, and 24 months), and trained research nurses familiar with infant health outcomes administered all questionnaires to minimize recall bias.
Patient Consent and Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committees and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The Southampton and South West Hampshire Research Ethics Committee approved all procedures (approval nos. 276/97 and 307/97). Informed consent was obtained from all individual participants included in the study.
Research Reporting Checklist
The STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guideline checklist (available at: http://www.strobe-statement.org/index.php?id=strobe-home) was used in the preparation of the manuscript.
DNA Extraction
A 5–10-cm segment was cut from the mid portion of each cord, immediately following delivery; flushed with saline to remove fetal blood; flash frozen in liquid nitrogen; and stored at -80°C until required for DNA isolation. Genomic DNA was prepared from umbilical cord and cord blood by a standard high salt method [14], and from adipose tissue using the QIAamp DNA mini kit (Qiagen, Hilden, Germany).
Genotyping of FUT2 Variants
A total of 1831 offspring and their mothers from the SWS cohort with infant health outcomes and available DNA specimens were genotyped for 2 FUT2 polymorphisms, rs601338 and rs602662, located on chromosome 19. The genomic region containing rs601338 and rs602662 was amplified by polymerase chain reaction analysis in accordance with the manufacturer’s guidelines (HotStarTaq Plus DNA Polymerase [catalog no. 203605], Qiagen; Supplementary Table 1). SNP genotyping was performed on a Pyromark MD instrument.
Statistical Analysis
Statistical procedures were performed in Stata, version 13 (StataCorp; College Station, TX), and SPSS, version 21 (IBM; Armonk, NY). The small number of offspring (n = 83) whose mothers were recorded as having an ethnicity other than white were excluded from the analysis, to ensure genetic homogeneity in the study population. Genotypes were coded according to the additive model (0, 1, or 2 copies of the G allele). Infant health outcomes were coded as dichotomous variables indicating whether the child had had =1 or no episode of the health outcome.
Binary regressions using each genotype individually used as a predictor variable for each infant health outcome were performed, with adjustment for child’s sex, parity, delivery mode (vaginal or cesarean section), maternal socioeconomic status, and breastfeeding duration split into 3 groups (never, =3 months, and >3 months). Analyses of infant diarrhea, from ages 6 to 12 months and from ages 12 to 24 months (which do not commonly result in a physician’s prescription of antibiotics) were additionally adjusted for infant antibiotic use, to reduce the likelihood of any observed associations resulting from antibiotic use rather than an infective episode. Infant antibiotic use from 6 to 12 months of age was also examined to see whether it was associated with episodes of diarrhea from ages 12 to 24 months. Regressions showing an association between offspring FUT2 SNPs and health outcomes were additionally adjusted for matching maternal genotype, when available, and for preterm birth (<37 weeks). Results from binary regression are expressed as risk ratios per unit increase in the number of risk alleles. A Benjamini-Hochberg procedure with a false-discovery rate of 10% was used to account for multiple testing.
RESULTS
Supplementary Table 3 shows genotype distributions for infants of white and other ethnicities in the SWS cohort. .2 tests show that there were associations between rs602662 and ethnicity (P = .004) and between rs601338 and ethnicity (P = .025). Expected values show that white infants had fewer g/g genotypes and more a/a genotypes than individuals of other ethnicities. Therefore, analysis was restricted to white infants, only because of insufficient numbers of infants of other ethnicities in the SWS cohort.
Both FUT2 SNPs, rs601338 and rs602662, were in Hardy-Weinberg equilibrium in the infants with white ethnicity (P > .05, by .2 analysis). rs601338 had a minor allele frequency of 48.7% (A), and rs602662 had a minor allele frequency of 48.6% (G). rs602662 is in high linkage disequilibrium with rs601338 in white populations (R2 = 0.92; Ensembl).
Analysis of infant health outcomes was based on 1831 SWS singleton births with available phenotype data from birth to 24 months of age and genotype data for at least 1 SNP. Table 1 shows summary statistics for the study sample from the SWS cohort (n = 1831). A comparison of 1831 infants who were and 1327 who were not included in the study is shown in Supplementary Table 4.
Figure 1 shows risk ratios and 95% confidence intervals (CIs) for each copy of the risk allele (G) for rs601338 and all infant health outcomes tested.
Table 1. Summary Statistics for the Study Sample 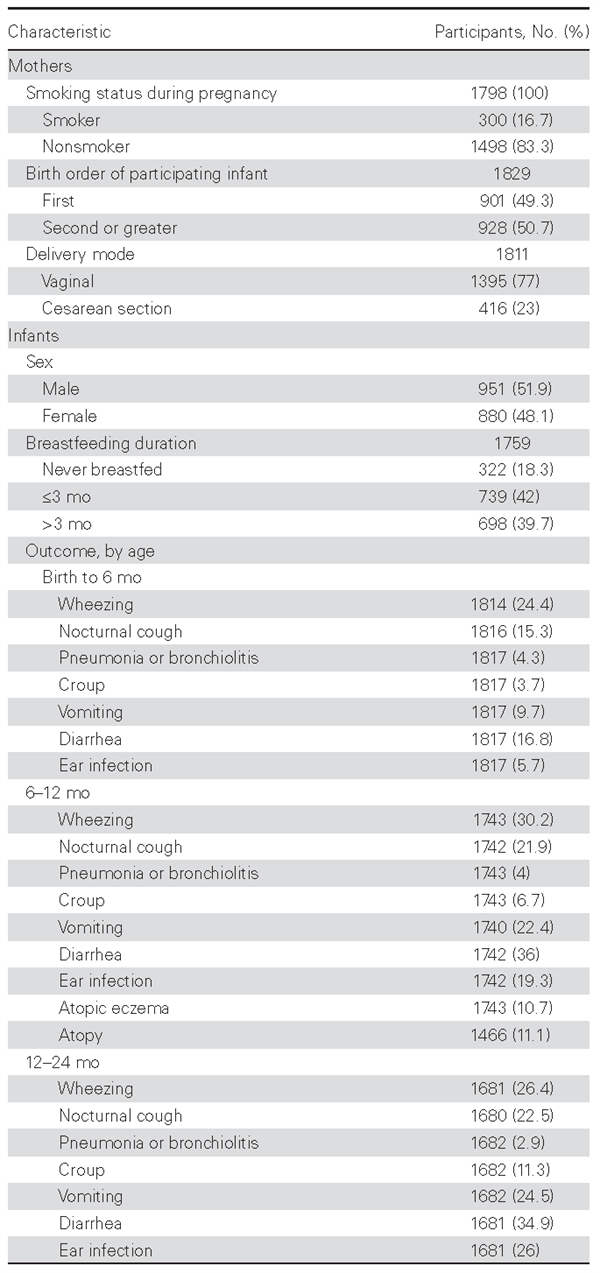
For FUT2 SNP rs601338, infants who possessed the G allele were more likely to experience vomiting, diarrhea, pneumo-nia/bronchiolitis, and nocturnal cough than infants possessing A alleles. Thus, FUT2 SNP rs601338 showed a significant association with =1 episode of diarrhea from birth to 6 months of age (risk ratio, 1.20 [95% CI, 1.03–1.4]; P = .017), 6 to 12 months of age (risk ratio, 1.15 [95% CI, 1.04–1.26]; P = .004), and 12 to 24 months of age (risk ratio, 1.29 [95% CI, 1.17–1.43]; P = 3.27 ×10-7). rs601338 was associated with =1 episode of vomiting from 6 to 12 months of age (risk ratio, 1.23 [95% CI, 1.08– 1.4]; P = .002) and from 12 to 24 months of age (risk ratio, 1.41 [95% CI, 1.24–1.61]; P = 1.7 × 10-7). rs601338 was also associated with =1 diagnosis of a lower respiratory tract illness (ie, pneumonia or bronchiolitis) between 12 and 24 months of age (risk ratio, 2.66 [95% CI, 1.64–4.3]; P = .00007) and with nocturnal cough between 12 and 24 months of age (risk ratio, 1.16 [95% CI, 1.02–1.33]; P = .029). No significant associations were observed between rs601338 and croup, wheezing, ear infections, atopic eczema, or atopy from birth to age 24 months (Figure 1). Similar results were observed for rs602662 because the 2 SNPs are in high linkage disequilibrium (R2 = 0.92; Supplementary Figure 1).
Antibiotic use from 6 to 12 months of age was associated with increased episodes of diarrhea from ages 12 to 24 months (P = .006).
Length of breastfeeding (>3 months, compared with never having breastfed) was also a significant predictor of infant diarrhea and pneumonia/bronchiolitis from 12 to 24 months of age (P = .042). Longer breastfeeding duration lowered the risk of diarrhea and pneumonia/bronchiolitis, independent of infant FUT2 genotype. Maternal FUT2 genotype was not found to be a significant predictor of infant health outcomes when infant FUT2 genotype was included in the model. Preterm birth was not a significant predictor of infant health outcomes, with a Benjamini-Hochberg false-discovery rate of 10%.
DISCUSSION
Our study showed significant associations of FUT2 SNPs rs601338 and rs602662 with the risks of reported respiratory and diarrheal illnesses, as well as vomiting and nocturnal cough, from birth to 24 months of age in infants in a population-based study. The risk of diarrheal infections was significantly higher among infants possessing the G allele as compared to those with the A alleles in FUT2 SNPs. The result was consistent from birth to 6 months, to 12 months, and up to 24 months of age. Prevalence rates for =1 reported bout of diarrhea lasting >2 days were highest between 6 and 12 months of age, being reported for 36% of infants. Reported pneumonia or bronchiolitis was less frequent in this population, ranging from 2.9% between birth and age 6 months to 4.3% between ages 12 and 24 months. G alleles on both FUT2 SNPs tested conferred a higher risk of pneumonia or bronchiolitis in this population. Other comorbidities potentially linked to infectious diseases, like vomiting lasting >2 days and nocturnal cough, were also associated with these FUT2 SNPs at ages 6 to 12 months and 24 months, respectively. The direction of the association was the same, with a lower risk of illness in the carriers of G alleles.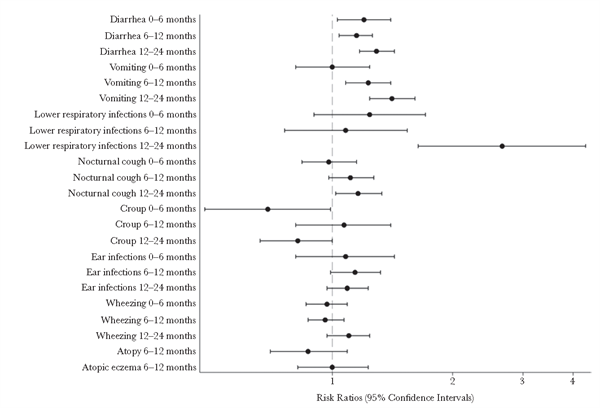
Figure 1. Risk ratios and 95% confidence intervals for each copy of the risk allele (G) for the FUT2 single-nucleotide polymorphism rs601338 and all health outcomes tested among infants, by age. The dashed line shows the risk ratio of the null effect; 95% confidence intervals crossing this line show that the outcome is not associated with rs601338.
Our main findings are in line with existing literature on diarrheal infections and host genetic susceptibility. FUT2 genetic variations have been associated with susceptibility to diarrheal infections in several studies [9, 10, 15, 16]. SNP rs601338 (W154X) was identified as the possible causal variant in a recent meta-analysis of birth cohorts studies consisting of >5000 young children [9]. The strongest association was found for at least 1 episode of diarrhea reported around age 1 year, but strong results were also reported for other time points. Similarly, in our study, a reduced risk of diarrheal episodes with the rs601338 SNP was consistently reported from 6 to 24 months of age but with a stronger association for diarrheal episodes from 12 to 24 months of age. A community-based birth study from Ecuador assessed the associations of norovirus gastroenteritis with secretor status during the first 3 years of life and identified that secretor children were more susceptible to norovirus GII.4 genotype infections, whereas nonsecretors were susceptible to non-GII.4 infections [17]. Results of another study, in China, of children with acute diarrhea were in line in these findings, although the detection of cases of norovirus GII.4- and GII.3associated diarrheal infections in nonsecretors showed that these children are not fully protected [18]. Our data did not include information on the specific diarrheal etiology or identification of pathogens; however, it is known that norovirus and rotavirus infections are the commonest among children, and they are influenced by the FUT2 genotype [10, 16, 19]. Previous studies suggest that a direct association between the secretor phenotype or FUT2 genotypes and the incidence of norovirus- or rotavirus-identified infection outbreaks likely exists, with nonsecretor or carriers of FUT2 nonfunctional mutations having protection against these infections [10, 16, 19] (Figure 2). FUT2 genetic variations or secretor status, as parts of the innate host genetic factors, have been also shown to interact with intestinal microbiota [20–22]. In light of this, a recent study aimed to investigate the interactions among FUT2 genotypes, gut microbiota, and viral infections typically linked to norovirus and rotavirus [23]. The study, however, could not confirm significant differences in intestinal microbial composition between FUT2 genotype groups, suggesting that these associations may be specific to the disease context or environmental exposures [24, 25]. Whether there is a direct link of association between risk of diarrheal infections and FUT2 genotypes or whether intestinal microbiota are interacting with host genetics to modulate risk of infections is not well understood.
Our novel findings on reported respiratory illnesses are in the same direction as in the recent study by Taylor et al, in which secretors with non–cystic fibrosis bronchiectasis had an increased susceptibility to Pseudomonas aeruginosa–dominated airway infection, compared with nonsecretors, among other disease outcomes [26]. It would be important to confirm these observations in future studies of children with known etiology of the respiratory infections and other comorbidities.
FUT2 is highly polymorphic, with different inactivating mutations in particular ethnic groups, but this genetic heterogeneity gives rise to similar proportions of the nonsecre-tor phenotype; for example, approximately 20% of Europeans and 22% of East Asians are nonsecretors [27]. American populations of Hispanic ancestry are less likely to be nonsecre-tors, compared with non-Hispanic populations, highlighting the low prevalence of the FUT2-inactivating mutations in Amerindian populations [15]. Nonsecretors are characterized by an absence of H antigen in salivary secretions and on mucosal surfaces of the body. In European and African populations, the most common inactivating variant is rs601338 (W154X), whereas in Asian populations the missense SNP rs1047781 (I140F) leads to a truncated protein with weak bioactivity [28]. The G risk allele in our study of rs601338 encodes the secretor phenotype, whereas the A allele encodes the nonsecretor phenotype. The second FUT2 variant included in our analysis, rs602662, is a missense SNP (G258S) in high linkage disequilibrium with rs601338, as shown in our results. This SNP was included in our study, as it has been shown to lead independently of rs601338 to the expression of a FUT2 enzyme with very low activity and thus might influence the expression of H antigen, as well [29]. HBGA groups like the H antigen may mediate the attachment of pathogens, leading to infection. In the case of rotavirus, the cell attachment viral spike protein VP8* can recognize A-type HBGA, perhaps leading to increased susceptibility of secretors as compared to nonsecretors to specific rotavirus strains [30, 31].
Our study showed an association of FUT2 SNPs with reported pneumonia or bronchiolitis and nocturnal cough from ages 12 to 24 months. A single previous study reported that secre-tors had increased susceptibility to respiratory infections with influenza viruses A and B, rhinoviruses, respiratory syncytial viruses, and echoviruses, but further evidence for the role of the FUT2 genetic polymorphisms in respiratory illnesses is scarce, with none in population-based studies [32]. We acknowledge with caution the findings on reported pneumonia or bronchiolitis, because the number of episodes in our population within the specific periods was low (Table 1), but a clear association was nonetheless observed. 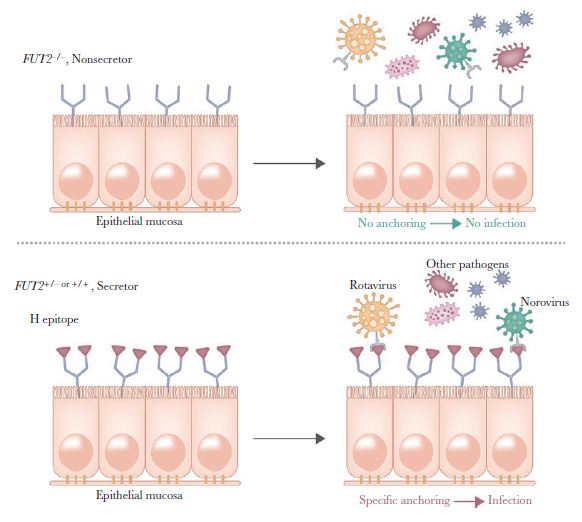
Figure 2. The proposed mechanism of interaction between the H epitope defned by FUT2 and pathogens. FUT2 inactivating mutations (rs601338 [W154X] and rs1047781
[I140F]) leading to the nonsecretor phenotype are associated with reduced susceptibility to infections.
Breastfeeding was identified as an independent protective exposure in our results, raising the possibility that some of the factors in human milk may reduce the risk of infections independently. The FUT2 enzyme also controls the production of specific human milk oligosaccharides by adding a fucose molecule to lactose in an a1,2-fucosylation activity to generate more-complex oligosaccharides, such as 2´-fucolsyllactose and lacto-N-fucosylpentaose I. These human milk oligosaccharides are present in the milk of all secretor mothers and are absent in the milk of nonsecretor mothers. Previous studies have demonstrated a specific protective effect of these oligosaccharides against diarrheal infections in a cohort of Mexican mothers and infants [33]. In that study, where infants were predominantly breastfed, it was postulated that the presence of these indigested oligosaccharides could compete with the attachment of pathogens to the intestinal epithelial surfaces by inhibiting their colonization and subsequent infection. By using this paradigm, a hypothesis could be proposed that the presence of these a1,2-fucosylated human milk oligosaccharides provided by a secretor lactating mother to her secretor infant might attenuate the increased risk that these infants inherently have to specific strains of viruses (eg, rotavirus or norovirus), considering that the glycan structures present in both human milk oligosaccharides and the epithelial epitopes are similar. Our study examined the association of maternal secretor status in relation with the risk of infections but no independent effect was found. However, many of the population of mothers and infants we studied were not exclusively breastfed and samples were not available to analyze the presence of oligosaccharides in the mother’s breast milk. We might also expect differences in the causal pathogens between our study and the previous Mexican study [33]. Future studies will need to assess this hypothesis by determining infant secretor status and sequencing for FUT2 variants, as well as analyzing the presence of a1,2-fucosylated human milk oligosaccharides in a predominantly breastfed population.
The main strength of our study was the relatively large population, which provided statistical power to examine a number of associations with FUT2 genotypes. The main limitations are, first, that information was not available on the etiology of the illnesses, and available data on both reported illnesses and genotyping information were available only for a part and not the entire SWS cohort [13]. Despite the large size of our population, we also did not have the power to analyze subpopulations with different ethnicity. Infant outcomes were self-reported by the mothers through questionnaires administered by research nurses and could possibly be affected by recall bias; this was minimized, however, by the short intervals between visits and by trained research nurses administering the questionnaires. Despite this lack of phenotypic specificity, we identified significant associations, consistent through time and in the same direction. Second, we could not accurately measure the level of exposure to pathogens because our study could assess neither the level of exposure to these pathogens in the population nor variations in hygiene between the FUT2 subgroups analyzed. Our study has highlighted the consistent association of FUT2 variants with the risk of diarrhea in infants and has described novel associations with respiratory illnesses and related comorbidities in a large population of infants. Host genetic susceptibility to infections needs to be accounted for in future epidemiological and interventional studies investigating these outcomes, alongside evaluation of the protective role of early life factors such as breastfeeding.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. This work was supported by Nestec, alone and under a research agreement with the University of Southampton, Auckland UniServices, Singapore Institute for Clinical Sciences, National University Hospital Singapore, and the National University of Singapore; the UK Medical Research Council (to the Southampton Women’s Survey, as part of an MRC award to the MRC Lifecourse Epidemiology Unit; and award MC_UU_12011/4 to K. M. G.); the British Heart Foundation (to the Southampton Women’s Survey); Food Standards Agency (to the Southampton Women’s Survey); Arthritis Research UK (to the Southampton Women’s Survey); the National Osteoporosis Society (to the Southampton Women’s Survey); the International Osteoporosis Foundation (to the Southampton Women’s Survey); the Cohen Trust (to the Southampton Women’s Survey); the National Institute for Health Research (NIHR) Senior Investigator Award: NF-SI-0515-10042 to K. M. G. as an NIHR senior investigator and through the NIHR Southampton Biomedical Research Centre: IS-BRC-1215-20004; and the European Union Erasmus+ Capacity Building in Higher Education “Early Nutrition e-Academy Southeast Asia”: 573651-EPP-1-2016-1-DE-EPPKA2-CBHE-JP to K. M. G., the European Union FP7 Project “EarlyNutrition”: Grant agreement number 289346, the European Union FP7 Project “ODIN”: Grant agreement number 613977.
Potential conflict of interest. K. M. G. has received reimbursement for speaking at conferences sponsored by companies selling nutritional products and is part of an academic consortium that has received research funding from Abbott Nutrition, Nestec, and Danone. I. S. Z., N. S., and A. B. are employees of Nestec. S. J. B., R. M., and K. A. L. are part of an academic consortium that has received research funding from Abbott Nutrition, Nestec, and Danone. All other authors: No reported conflicts.
References
- Walker CLF, Rudan I, Liu L, et al. Global burden of childhood pneumonia and diarrhoea. Lancet 2013; 381:1405–16.
- Keusch GT, Walker CF, Das JK, Horton S, Habte D. Diarrheal diseases. In: Black RE, Laxminarayan R, Temmerman M, Walker N, eds. Reproductive, maternal, newborn, and child health: disease control priorities. 3rd ed. Vol 2. Washington, DC: World Bank Publications, 2016.
- Shi T, McAllister DA, O’Brien KL, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet 2017; 390:946–58.
- Fischer Walker CL, Perin J, Aryee MJ, Boschi-Pinto C, Black RE. Diarrhea incidence in low- and middle-income countries in 1990 and 2010: a systematic review. BMC Public Health 2012; 12:220.
- Wiegering V, Kaiser J, Tappe D, Weissbrich B, Morbach H, Girschick HJ. Gastroenteritis in childhood: a retrospective study of 650 hospitalized pediatric patients. Int J Infect Dis 2011; 15:e401–7.
- Nair H, Nokes DJ, Gessner BD, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 2010; 375:1545–55.
- Duijts L, Jaddoe VW, Hofman A, Moll HA. Prolonged and exclusive breastfeeding reduces the risk of infectious diseases in infancy. Pediatrics 2010; 126:e18–25.
- Prameela KK, Vijaya LR. The importance of breastfeeding in rotaviral diarrhoeas. Malays J Nutr 2012; 18:103–11.
- Bustamante M, Standl M, Bassat Q, et al. A genome-wide association meta-analysis of diarrhoeal disease in young children identifies FUT2 locus and provides plausible biological pathways. Hum Mol Genet 2016; 25:4127–42.
- Kindberg E, Akerlind B, Johnsen C, et al. Host genetic resistance to symptomatic norovirus (GGII.4) infections in Denmark. J Clin Microbiol 2007; 45:2720–2.
- Kambhampati A, Payne DC, Costantini V, Lopman BA. Host Genetic Susceptibility to Enteric Viruses: A Systematic Review and Metaanalysis. Clin Infect Dis 2016; 62:11–8.
- Koda Y, Tachida H, Pang H, et al. Contrasting patterns of polymorphisms at the ABO-secretor gene (FUT2) and plasma alpha(1,3)fucosyltransferase gene (FUT6) in human populations. Genetics 2001; 158:747–56.
- Inskip HM, Godfrey KM, Robinson SM, Law CM, Barker DJ, Cooper C; SWS Study Group. Cohort profile: the Southampton Women’s Survey. Int J Epidemiol 2006; 35:42–8.
- Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res 1988; 16:1215.
- Payne DC, Currier RL, Staat MA, et al. Epidemiologic association between FUT2 secretor status and severe rotavirus gastroenteritis in children in the United States. JAMA Pediatr 2015; 169:1040–5.
- Thorven M, Grahn A, Hedlund KO, et al. A homozygous nonsense mutation (428G–>A) in the human secretor (FUT2) gene provides resistance to symptomatic norovirus (GGII) infections. J Virol 2005; 79:15351–5.
- Lopman BA, Trivedi T, Vicuña Y, et al. Norovirus infection and disease in an ecuadorian birth cohort: association of certain norovirus genotypes with host FUT2 secretor status. J Infect Dis 2015; 211:1813–21.
- Liu P, Wang X, Lee JC, et al. Genetic susceptibility to noro-virus GII.3 and GII.4 infections in Chinese pediatric diarrheal disease. Pediatr Infect Dis J 2014; 33:e305–9.
- Imbert-Marcille BM, Barbé L, Dupé M, et al. A FUT2 gene common polymorphism determines resistance to rotavirus A of the P[8] genotype. J Infect Dis 2014; 209:1227–30.
- Wacklin P, Tuimala J, Nikkilä J, et al. Faecal microbiota composition in adults is associated with the FUT2 gene determining the secretor status. PLoS One 2014; 9:e94863.
- Wacklin P, Mäkivuokko H, Alakulppi N, et al. Secretor genotype (FUT2 gene) is strongly associated with the composition of Bifidobacteria in the human intestine. PLoS One 2011; 6:e20113.
- Kolde R, Franzosa EA, Rahnavard G, et al. Host genetic variation and its microbiome interactions within the Human Microbiome Project. Genome Med 2018; 10:6.
- Rodríguez-Díaz J, García-Mantrana I, Vila-Vicent S, et al. Relevance of secretor status genotype and microbiota composition in susceptibility to rotavirus and norovirus infections in humans. Sci Rep 2017; 7:45559.
- Davenport ER, Goodrich JK, Bell JT, Spector TD, Ley RE, Clark AG. ABO antigen and secretor statuses are not associated with gut microbiota composition in 1,500 twins. BMC Genomics 2016; 17:941.
- Rausch P, Rehman A, Künzel S, et al. Colonic mucosa-associated microbiota is influenced by an interaction of Crohn disease and FUT2 (Secretor) genotype. Proc Natl Acad Sci U S A 2011; 108:19030–5.
- Taylor SL, Woodman RJ, Chen AC, et al. FUT2 genotype influences lung function, exacerbation frequency and airway microbiota in non-CF bronchiectasis. Thorax 2017; 72:304–10.
- Ferrer-Admetlla A, Sikora M, Laayouni H, et al. A natural history of FUT2 polymorphism in humans. Mol Biol Evol 2009; 26:1993–2003.
- Silva LM, Carvalho AS, Guillon P, et al. Infection-associated FUT2 (Fucosyltransferase 2) genetic variation and impact on functionality assessed by in vivo studies. Glycoconj J 2010; 27:61–8.
- Serpa J, Mendes N, Reis CA, et al. Two new FUT2 (fucosyltransferase 2 gene) missense polymorphisms, 739G–>A and 839T–>C, are partly responsible for non-secretor status in a Caucasian population from Northern Portugal. Biochem J 2004; 383: 469–74.
- Hu L, Crawford SE, Czako R, et al. Cell attachment protein VP8* of a human rotavirus specifically interacts with A-type histo-blood group antigen. Nature 2012; 485:256–9.
- Liu Y, Huang P, Tan M, et al. Rotavirus VP8*: phylogeny, host range, and interaction with histo-blood group antigens. J Virol 2012; 86:9899–910.
- Raza MW, Blackwell CC, Molyneaux P, et al. Association between secretor status and respiratory viral illness. BMJ (Clinical research ed) 1991; 303:815–8.
- Morrow AL, Ruiz-Palacios GM, Jiang X, Newburg DS. Human-milk glycans that inhibit pathogen binding protect breast-feeding infants against infectious diarrhea. J Nutr 2005; 135:1304–7.
If you liked this post you may also like